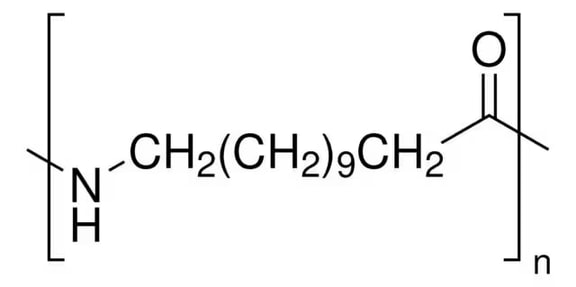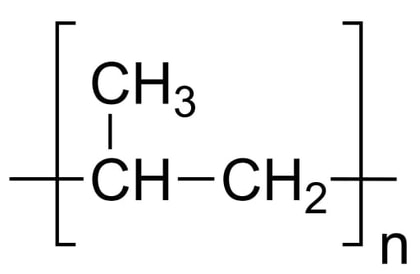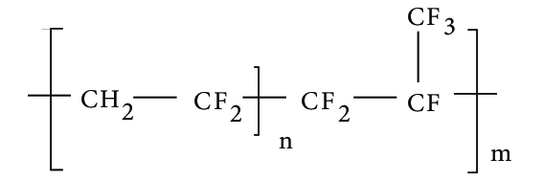Center for Pharmaceutical Cleaning Innovation
Materials of Construction
This page contains information about the various Materials of Construction (MoC) used in the Pharmaceutical, Biotech and Medical Device industries that CPCI™ provides coupons for. Quite often, customers want to purchase coupons but are unsure of the exact MoC they need. CPCI™ has provided this webpage for visitors to reference when they are interested in purchasing coupons from us, but may be unsure of the exact MoC they need.
CPCI™ has compiled this information from the various material suppliers' webpages and from Wikipedia. We hope this information helps you identify the right MoC for your coupons. Once you have identified the right MoC, you can click the link in the Material name and it will take you to coupons made from that MoC in the CPCI™ Webstore.
CPCI™ has compiled this information from the various material suppliers' webpages and from Wikipedia. We hope this information helps you identify the right MoC for your coupons. Once you have identified the right MoC, you can click the link in the Material name and it will take you to coupons made from that MoC in the CPCI™ Webstore.
304 Stainless Steel/#4 Finish / EN Designation 1J-2J
NOTE: Use the link above in the coupon name to go to the product page to purchase.
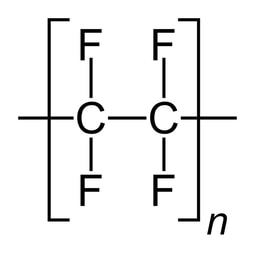
Description: 304 Stainless Steel with a #4 Finish and EN Designation 1J-2J is a highly corrosion-resistant and versatile material widely employed in various industries, including pharmaceuticals. The EN Designation 1J-2J signifies Brushed or dull polished. 304 stainless steel is the most common stainless steel. 304 contains both chromium (between 18% and 20%) and nickel (between 8% and 10.5%) metals as the main non-iron constituents. 304 is an austenitic stainless steel. It is less electrically and thermally conductive than carbon steel. 304 is magnetic, but less magnetic than steel. It has a higher corrosion resistance than regular steel and is widely used because of the ease in which it is formed into various shapes.
Pharmaceutical Uses: In the pharmaceutical sector, 304/#4 finish stainless steel grade is used to fabricate equipment (e.g., glove boxes), containers, and components that are not "product-contact" but demand high levels of hygiene and resistance to chemicals. The #4 finish provides a smooth, satin-like surface, which is easier to clean and maintain in sterile environments, making it ideal for pharmaceutical processing equipment. It has a unidirectional texture and is not very reflective.
Beyond pharmaceuticals, 304 Stainless Steel is also employed in food processing, medical devices, and construction, where its corrosion resistance, durability, and ease of fabrication contribute to its widespread use.
NOTE: Use the link above in the coupon name to go to the product page to purchase.
Description: 304 Stainless Steel with a #4 Finish and EN Designation 1J-2J is a highly corrosion-resistant and versatile material widely employed in various industries, including pharmaceuticals. The EN Designation 1J-2J signifies Brushed or dull polished. 304 stainless steel is the most common stainless steel. 304 contains both chromium (between 18% and 20%) and nickel (between 8% and 10.5%) metals as the main non-iron constituents. 304 is an austenitic stainless steel. It is less electrically and thermally conductive than carbon steel. 304 is magnetic, but less magnetic than steel. It has a higher corrosion resistance than regular steel and is widely used because of the ease in which it is formed into various shapes.
Pharmaceutical Uses: In the pharmaceutical sector, 304/#4 finish stainless steel grade is used to fabricate equipment (e.g., glove boxes), containers, and components that are not "product-contact" but demand high levels of hygiene and resistance to chemicals. The #4 finish provides a smooth, satin-like surface, which is easier to clean and maintain in sterile environments, making it ideal for pharmaceutical processing equipment. It has a unidirectional texture and is not very reflective.
Beyond pharmaceuticals, 304 Stainless Steel is also employed in food processing, medical devices, and construction, where its corrosion resistance, durability, and ease of fabrication contribute to its widespread use.
316L/#4 Finish / EN Designation 1J-2J
NOTE: Use the link above in the coupon name to go to the product page to purchase.
Description: 316L Stainless Steel with a #4 Finish and EN Designation 1J-2J is a premium-grade stainless steel material with exceptional corrosion resistance and a smooth, satin-like surface finish, making it highly suitable for demanding applications, including pharmaceuticals. The EN Designation 1J-2J signifies Brushed or dull polished. 316 is sometimes referred to as A4 stainless steel or marine grade stainless steel, is the second most common austenitic stainless steel after 304/A2 stainless steel. Its primary alloying constituents after iron, are chromium (between 16–18%), nickel (10–12%) and molybdenum (2–3%), with small (<1%) quantities of silicon, phosphorus & sulfur also present. The addition of molybdenum provides greater corrosion resistance than 304, with respect to localized corrosive attack by chlorides and to general corrosion by reducing acids, such as sulfuric acid.[1] 316L grade is the low carbon version of 316 stainless steel. When cold worked, 316 can produce high yield and tensile strengths similar to Duplex stainless grades.
Pharmaceutical Uses: In the pharmaceutical industry, 316L stainless steel is a preferred choice for manufacturing critical components like tanks, vessels, and piping systems due to its resistance to chemical corrosion and ability to maintain product purity. The #4 finish not only enhances its appearance but also simplifies cleaning and maintenance in sterile environments. It has a unidirectional texture and is not very reflective.
Beyond pharmaceuticals, 316L Stainless Steel is utilised in industries such as biotechnology, food processing, and medical devices, where its corrosion resistance and biocompatibility are crucial for maintaining product quality and safety.
NOTE: Use the link above in the coupon name to go to the product page to purchase.
Description: 316L Stainless Steel with a #4 Finish and EN Designation 1J-2J is a premium-grade stainless steel material with exceptional corrosion resistance and a smooth, satin-like surface finish, making it highly suitable for demanding applications, including pharmaceuticals. The EN Designation 1J-2J signifies Brushed or dull polished. 316 is sometimes referred to as A4 stainless steel or marine grade stainless steel, is the second most common austenitic stainless steel after 304/A2 stainless steel. Its primary alloying constituents after iron, are chromium (between 16–18%), nickel (10–12%) and molybdenum (2–3%), with small (<1%) quantities of silicon, phosphorus & sulfur also present. The addition of molybdenum provides greater corrosion resistance than 304, with respect to localized corrosive attack by chlorides and to general corrosion by reducing acids, such as sulfuric acid.[1] 316L grade is the low carbon version of 316 stainless steel. When cold worked, 316 can produce high yield and tensile strengths similar to Duplex stainless grades.
Pharmaceutical Uses: In the pharmaceutical industry, 316L stainless steel is a preferred choice for manufacturing critical components like tanks, vessels, and piping systems due to its resistance to chemical corrosion and ability to maintain product purity. The #4 finish not only enhances its appearance but also simplifies cleaning and maintenance in sterile environments. It has a unidirectional texture and is not very reflective.
Beyond pharmaceuticals, 316L Stainless Steel is utilised in industries such as biotechnology, food processing, and medical devices, where its corrosion resistance and biocompatibility are crucial for maintaining product quality and safety.
316L/#8 Finish / EN Designation 1P-2P
NOTE: Use the link above in the coupon name to go to the product page to purchase.
Description: 316L Stainless Steel with a #8 Finish and EN Designation 1P-2P represents a high-quality stainless steel material with impeccable corrosion resistance and an ultra-smooth, mirror-like surface finish, making it exceptionally suitable for demanding applications, such as Biopharmaceuticals. The EN Designation 1P-2P means it is Bright polished. 1P-2P has a non-directional finish, reflective with a high degree of image clarity.
Pharmaceutical Uses: In the pharmaceutical industry, 316L stainless steel is the material of choice for fabricating critical components such as storage vessels, bioreactors, and other Biopharmaceutical processing equipment, due to its superior resistance to chemical corrosion and ability to uphold product purity. The #8 finish facilitates ease of cleaning and disinfection in sterile environments, making it preferred for Biopharmaceutical applications.
Beyond Biopharmaceuticals, 316L Stainless Steel with a #8 finish finds usage in medical devices, and high-purity applications, where corrosion resistance and a pristine surface finish are essential to .
NOTE: Use the link above in the coupon name to go to the product page to purchase.
Description: 316L Stainless Steel with a #8 Finish and EN Designation 1P-2P represents a high-quality stainless steel material with impeccable corrosion resistance and an ultra-smooth, mirror-like surface finish, making it exceptionally suitable for demanding applications, such as Biopharmaceuticals. The EN Designation 1P-2P means it is Bright polished. 1P-2P has a non-directional finish, reflective with a high degree of image clarity.
Pharmaceutical Uses: In the pharmaceutical industry, 316L stainless steel is the material of choice for fabricating critical components such as storage vessels, bioreactors, and other Biopharmaceutical processing equipment, due to its superior resistance to chemical corrosion and ability to uphold product purity. The #8 finish facilitates ease of cleaning and disinfection in sterile environments, making it preferred for Biopharmaceutical applications.
Beyond Biopharmaceuticals, 316L Stainless Steel with a #8 finish finds usage in medical devices, and high-purity applications, where corrosion resistance and a pristine surface finish are essential to .
316L/Electropolished
Description: 316L Stainless Steel with an electropolished finish is a premium-grade material that combines the excellent corrosion resistance of 316L stainless steel with a refined surface treatment.
It is important to understand what is actually meant when the term "electropolished" is used. Electropolishing, also known as electrochemical polishing, anodic polishing, or electrolytic polishing, is an electrochemical process that removes material from a metallic workpiece, reducing the surface roughness (Ra) by leveling micro-peaks and valleys, improving the surface finish (Wikipedia). However, the process of electropolishing can only reduce the Ra of the metal by about 50%. So a piece of Stainless Steel that has a starting Ra of 50 μm will only be reduced to about 25 μm after electropolishing. So the Ra of the starting material is important for obtaining the desired Ra of the electropolished item. Depending on the starting material used, there can be many different levels of surface roughness after electropolishing. However, there are some standards available such as the American Society of Mechanical Engineering BioProcessing Equipment Guide (ASME BPE). The ASME BPE has 6 Surface Designations (SF0 to SF6) with SF4 being the smoothest designation (Ra ≤ 0.38 μm) and this is the designation that is most commonly used in pharmaceutical applications.
Pharmaceutical Uses: In pharmaceutical applications, 316L stainless steel with is often chosen for critical components such as tanks, piping, and pharmaceutical processing equipment. Electropolishing enhances the material's surface by removing microscopic imperfections, improving its resistance to corrosion, reducing areas where bacteria can hide and start a biofilm and making it easier to clean. This is particularly beneficial in pharmaceutical cleanroom environments where hygiene is of the utmost importance. The electropolished finish ensures the highest levels of surface purity, making it ideal for applications requiring exceptional cleanliness and sterilization.
Beyond pharmaceuticals, this material is used in biotechnology, medical devices, and high-purity applications where corrosion resistance and surface quality are vital for product integrity and safety.
Description: 316L Stainless Steel with an electropolished finish is a premium-grade material that combines the excellent corrosion resistance of 316L stainless steel with a refined surface treatment.
It is important to understand what is actually meant when the term "electropolished" is used. Electropolishing, also known as electrochemical polishing, anodic polishing, or electrolytic polishing, is an electrochemical process that removes material from a metallic workpiece, reducing the surface roughness (Ra) by leveling micro-peaks and valleys, improving the surface finish (Wikipedia). However, the process of electropolishing can only reduce the Ra of the metal by about 50%. So a piece of Stainless Steel that has a starting Ra of 50 μm will only be reduced to about 25 μm after electropolishing. So the Ra of the starting material is important for obtaining the desired Ra of the electropolished item. Depending on the starting material used, there can be many different levels of surface roughness after electropolishing. However, there are some standards available such as the American Society of Mechanical Engineering BioProcessing Equipment Guide (ASME BPE). The ASME BPE has 6 Surface Designations (SF0 to SF6) with SF4 being the smoothest designation (Ra ≤ 0.38 μm) and this is the designation that is most commonly used in pharmaceutical applications.
Pharmaceutical Uses: In pharmaceutical applications, 316L stainless steel with is often chosen for critical components such as tanks, piping, and pharmaceutical processing equipment. Electropolishing enhances the material's surface by removing microscopic imperfections, improving its resistance to corrosion, reducing areas where bacteria can hide and start a biofilm and making it easier to clean. This is particularly beneficial in pharmaceutical cleanroom environments where hygiene is of the utmost importance. The electropolished finish ensures the highest levels of surface purity, making it ideal for applications requiring exceptional cleanliness and sterilization.
Beyond pharmaceuticals, this material is used in biotechnology, medical devices, and high-purity applications where corrosion resistance and surface quality are vital for product integrity and safety.
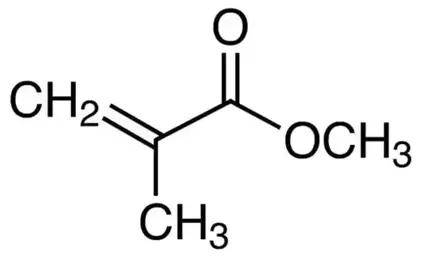
Acrylic
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Poly(methyl methacrylate) (PMMA) is a synthetic polymer derived from methyl methacrylate. It is a transparent thermoplastic and serves as an engineering plastic with a wide range of applications. PMMA is produced through various methods, including emulsion polymerization, solution polymerization, and bulk polymerization. These processes typically employ radical initiation, but anionic polymerization can also be used for PMMA production.
PMMA is recognized by several names, such as acrylic, acrylic glass, and various trade names and brands like Crylux, Hesalite, Plexiglas, Acrylite, Lucite, and Perspex, among others. This versatile plastic is commonly used in sheet form as a lightweight and shatter-resistant alternative to traditional glass. It also finds applications as a casting resin, in inks, coatings, and various other purposes.
PMMA offers a combination of advantageous properties, making it a popular choice:
- It is a strong, tough, and lightweight material.
- PMMA has a density ranging from 1.17 to 1.20 g/cm3, which is less than half that of glass.
- It exhibits good impact strength, surpassing both glass and polystyrene, though it falls short of the impact resistance of polycarbonate and certain engineered polymers.
- While PMMA can swell and dissolve in many organic solvents, it has limited resistance to certain chemicals due to the presence of easily hydrolyzed ester groups.
- However, PMMA excels in environmental stability compared to other plastics like polystyrene and polyethylene, making it a preferred material for outdoor applications.
PMMA's combination of transparency, strength, and versatility makes it a valuable material for a wide array of applications across various industries.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Poly(methyl methacrylate) (PMMA) is a synthetic polymer derived from methyl methacrylate. It is a transparent thermoplastic and serves as an engineering plastic with a wide range of applications. PMMA is produced through various methods, including emulsion polymerization, solution polymerization, and bulk polymerization. These processes typically employ radical initiation, but anionic polymerization can also be used for PMMA production.
PMMA is recognized by several names, such as acrylic, acrylic glass, and various trade names and brands like Crylux, Hesalite, Plexiglas, Acrylite, Lucite, and Perspex, among others. This versatile plastic is commonly used in sheet form as a lightweight and shatter-resistant alternative to traditional glass. It also finds applications as a casting resin, in inks, coatings, and various other purposes.
PMMA offers a combination of advantageous properties, making it a popular choice:
- It is a strong, tough, and lightweight material.
- PMMA has a density ranging from 1.17 to 1.20 g/cm3, which is less than half that of glass.
- It exhibits good impact strength, surpassing both glass and polystyrene, though it falls short of the impact resistance of polycarbonate and certain engineered polymers.
- While PMMA can swell and dissolve in many organic solvents, it has limited resistance to certain chemicals due to the presence of easily hydrolyzed ester groups.
- However, PMMA excels in environmental stability compared to other plastics like polystyrene and polyethylene, making it a preferred material for outdoor applications.
PMMA's combination of transparency, strength, and versatility makes it a valuable material for a wide array of applications across various industries.
Pharmaceutical Uses: In pharmaceuticals, acrylic's exceptional transparency and optical quality are harnessed for crafting precision components like cuvettes and lab equipment, ensuring accurate measurements and observations in pharmaceutical research and testing.
Beyond the pharmaceutical sector, acrylic is widely used in architectural and automotive applications, where its impact resistance and lightweight nature are valuable for windows, signage, and protective barriers. Its versatility and ease of fabrication have also made it a popular choice for creative and functional designs in retail displays and consumer goods.
Beyond the pharmaceutical sector, acrylic is widely used in architectural and automotive applications, where its impact resistance and lightweight nature are valuable for windows, signage, and protective barriers. Its versatility and ease of fabrication have also made it a popular choice for creative and functional designs in retail displays and consumer goods.
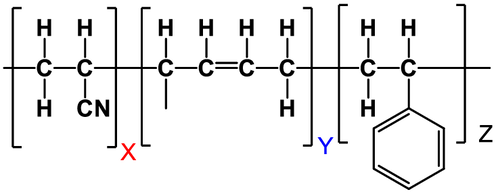
Acrylonitrile Butadiene Styrene (ABS)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Acrylonitrile butadiene styrene (ABS) is a common thermoplastic polymer with a chemical formula of (C8H8)x·(C4H6)y·(C3H3N)z. It exhibits unique properties in the world of plastics. ABS does not have a true melting point because it is amorphous in nature, but its glass transition temperature is approximately 105°C (221°F).
ABS is a terpolymer created through the polymerization of styrene, acrylonitrile, and polybutadiene. The proportions of these components can vary, typically ranging from 15% to 35% acrylonitrile, 5% to 30% butadiene, and 40% to 60% styrene. This results in a complex structure where long chains of polybutadiene are intertwined with shorter chains of poly(styrene-co-acrylonitrile). Each component contributes distinct properties to ABS:
- Acrylonitrile enhances chemical resistance, fatigue resistance, hardness, and rigidity, while also increasing the heat deflection temperature.
- Styrene provides the plastic with a shiny, impervious surface, as well as hardness, rigidity, and improved processing capabilities.
- Polybutadiene, a rubbery substance, imparts toughness and ductility at low temperatures, although it comes at the expense of heat resistance and rigidity.
ABS can be modified in various ways to improve impact resistance, toughness, and heat resistance. Increasing the proportion of polybutadiene relative to styrene and acrylonitrile enhances impact resistance, although it can affect other properties. Notably, the impact resistance of ABS remains stable at lower temperatures. ABS also demonstrates excellent stability under load with limited loads.
By altering the proportions of its components, ABS can be produced in different grades, each tailored for specific applications and performance characteristics.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Acrylonitrile butadiene styrene (ABS) is a common thermoplastic polymer with a chemical formula of (C8H8)x·(C4H6)y·(C3H3N)z. It exhibits unique properties in the world of plastics. ABS does not have a true melting point because it is amorphous in nature, but its glass transition temperature is approximately 105°C (221°F).
ABS is a terpolymer created through the polymerization of styrene, acrylonitrile, and polybutadiene. The proportions of these components can vary, typically ranging from 15% to 35% acrylonitrile, 5% to 30% butadiene, and 40% to 60% styrene. This results in a complex structure where long chains of polybutadiene are intertwined with shorter chains of poly(styrene-co-acrylonitrile). Each component contributes distinct properties to ABS:
- Acrylonitrile enhances chemical resistance, fatigue resistance, hardness, and rigidity, while also increasing the heat deflection temperature.
- Styrene provides the plastic with a shiny, impervious surface, as well as hardness, rigidity, and improved processing capabilities.
- Polybutadiene, a rubbery substance, imparts toughness and ductility at low temperatures, although it comes at the expense of heat resistance and rigidity.
ABS can be modified in various ways to improve impact resistance, toughness, and heat resistance. Increasing the proportion of polybutadiene relative to styrene and acrylonitrile enhances impact resistance, although it can affect other properties. Notably, the impact resistance of ABS remains stable at lower temperatures. ABS also demonstrates excellent stability under load with limited loads.
By altering the proportions of its components, ABS can be produced in different grades, each tailored for specific applications and performance characteristics.
Pharmaceutical Uses: In the pharmaceutical sector, ABS is employed for manufacturing a wide array of laboratory equipment, including beakers, pipette tips, and sample containers. Its robust and impact-resistant nature ensures the durability of these critical components, while its chemical resistance makes it suitable for use in pharmaceutical environments where contact with various substances is common. Additionally, ABS is valued for its ease of machining and cost-effectiveness in producing precision parts.
Beyond pharmaceuticals, ABS is extensively used in automotive, electronics, and consumer goods, where its combination of properties, including lightweight construction and affordability, makes it an excellent choice for various applications.
Beyond pharmaceuticals, ABS is extensively used in automotive, electronics, and consumer goods, where its combination of properties, including lightweight construction and affordability, makes it an excellent choice for various applications.
Alumina Ceramic
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Aluminium oxide, also known as aluminum(III) oxide, is a chemical compound composed of aluminum and oxygen, represented by the chemical formula Al2O3. It is the most frequently encountered among various aluminum oxides and is specifically referred to as aluminum oxide. Commonly, it is known as alumina and may be called by various names such as aloxide, aloxite, or alundum in different forms and applications.In its crystalline polymorphic phase known as α-Al2O3, aluminum oxide occurs naturally as the mineral corundum. Varieties of corundum are responsible for forming the precious gemstones ruby and sapphire.
Al2O3 serves a range of important purposes, including the production of aluminum metal, as an abrasive due to its hardness, and as a refractory material due to its high melting point. This compound is an electrical insulator but possesses relatively high thermal conductivity for a ceramic material. Importantly, aluminum oxide is insoluble in water. In its most prevalent crystalline form, known as corundum or α-aluminum oxide, its exceptional hardness makes it suitable for use as an abrasive and as a component in cutting tools.
Pharmaceutical Uses: Within the pharmaceutical industry, alumina ceramic plays a vital role in the production of specialized equipment and components. Its outstanding electrical insulation characteristics make it a preferred choice for manufacturing insulators and electrodes used in pharmaceutical instrumentation. Moreover, alumina ceramic's resistance to high temperatures and aggressive chemicals ensures its reliability in processes involving pharmaceutical compounds.
Beyond pharmaceuticals, this ceramic material is extensively used in the aerospace, electronics, and automotive industries for applications requiring extreme durability and resistance to wear, corrosion, and thermal stress. Alumina ceramic is also used to make a variety of other products, such as cutting tools, electronic components, and aerospace components.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Aluminium oxide, also known as aluminum(III) oxide, is a chemical compound composed of aluminum and oxygen, represented by the chemical formula Al2O3. It is the most frequently encountered among various aluminum oxides and is specifically referred to as aluminum oxide. Commonly, it is known as alumina and may be called by various names such as aloxide, aloxite, or alundum in different forms and applications.In its crystalline polymorphic phase known as α-Al2O3, aluminum oxide occurs naturally as the mineral corundum. Varieties of corundum are responsible for forming the precious gemstones ruby and sapphire.
Al2O3 serves a range of important purposes, including the production of aluminum metal, as an abrasive due to its hardness, and as a refractory material due to its high melting point. This compound is an electrical insulator but possesses relatively high thermal conductivity for a ceramic material. Importantly, aluminum oxide is insoluble in water. In its most prevalent crystalline form, known as corundum or α-aluminum oxide, its exceptional hardness makes it suitable for use as an abrasive and as a component in cutting tools.
Pharmaceutical Uses: Within the pharmaceutical industry, alumina ceramic plays a vital role in the production of specialized equipment and components. Its outstanding electrical insulation characteristics make it a preferred choice for manufacturing insulators and electrodes used in pharmaceutical instrumentation. Moreover, alumina ceramic's resistance to high temperatures and aggressive chemicals ensures its reliability in processes involving pharmaceutical compounds.
Beyond pharmaceuticals, this ceramic material is extensively used in the aerospace, electronics, and automotive industries for applications requiring extreme durability and resistance to wear, corrosion, and thermal stress. Alumina ceramic is also used to make a variety of other products, such as cutting tools, electronic components, and aerospace components.
Aluminum 6061
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: 6061 aluminum alloy, with the Unified Numbering System (UNS) designation A96061, is a precipitation-hardened aluminum alloy characterized by its major alloying elements, magnesium, and silicon. Initially known as "Alloy 61S," it was developed in 1935. This alloy boasts commendable mechanical properties, excellent weldability, and is frequently extruded, ranking as the second most popular alloy after 6063. It is widely employed for various general-purpose applications.The mechanical properties of 6061 aluminum alloy are significantly influenced by the temperature or heat treatment applied to the material. Young's Modulus for 6061 is a consistent 69 GPa (10,000 ksi), regardless of the specific treatment it undergoes.
Pharmaceutical Uses: In pharmaceuticals, Aluminum 6061 is utilized for manufacturing precision components and equipment, thanks to its lightweight nature, excellent machinability, and corrosion resistance. Its durability and ease of fabrication make it suitable for producing pharmaceutical processing equipment, ensuring reliable and efficient operations. Moreover, Aluminum 6061's biocompatibility and resistance to rust make it valuable for pharmaceutical cleanroom environments where hygiene is paramount.
Beyond pharmaceuticals, this aluminum alloy is extensively used in aerospace, automotive, and construction sectors, where its strength, versatility, and lightweight construction offer advantages in a wide range of applications.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: 6061 aluminum alloy, with the Unified Numbering System (UNS) designation A96061, is a precipitation-hardened aluminum alloy characterized by its major alloying elements, magnesium, and silicon. Initially known as "Alloy 61S," it was developed in 1935. This alloy boasts commendable mechanical properties, excellent weldability, and is frequently extruded, ranking as the second most popular alloy after 6063. It is widely employed for various general-purpose applications.The mechanical properties of 6061 aluminum alloy are significantly influenced by the temperature or heat treatment applied to the material. Young's Modulus for 6061 is a consistent 69 GPa (10,000 ksi), regardless of the specific treatment it undergoes.
Pharmaceutical Uses: In pharmaceuticals, Aluminum 6061 is utilized for manufacturing precision components and equipment, thanks to its lightweight nature, excellent machinability, and corrosion resistance. Its durability and ease of fabrication make it suitable for producing pharmaceutical processing equipment, ensuring reliable and efficient operations. Moreover, Aluminum 6061's biocompatibility and resistance to rust make it valuable for pharmaceutical cleanroom environments where hygiene is paramount.
Beyond pharmaceuticals, this aluminum alloy is extensively used in aerospace, automotive, and construction sectors, where its strength, versatility, and lightweight construction offer advantages in a wide range of applications.
Anodized Aluminum 6061
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Anodized aluminum 6061 is a type of aluminum 6061 that has been treated with an electrochemical process to create a hard, protective oxide coating on the surface. This coating makes the aluminum even more resistant to corrosion, wear, and abrasion. It also gives the aluminum a smooth, attractive finish that is easy to clean and maintain. Anodized Aluminum 6061, a specialized treatment of the versatile aluminum alloy, enhances its surface properties for many applications, including those in the pharmaceutical industry.
Pharmaceutical Uses: In pharmaceuticals, Anodized Aluminum 6061 is employed to manufacture components such as lab equipment, cabinets, and cleanroom fixtures. The anodizing process creates a protective oxide layer on the aluminum surface, providing superior corrosion resistance and improved durability. This makes it well-suited for pharmaceutical cleanrooms where maintaining a sterile environment is critical. The enhanced surface hardness of anodized Aluminum 6061 also ensures resistance to wear and abrasion, prolonging the lifespan of the equipment.
Beyond pharmaceuticals, this material finds applications in aerospace, electronics, and architectural projects, where its aesthetic appeal, corrosion resistance, and durability are highly valued.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Anodized aluminum 6061 is a type of aluminum 6061 that has been treated with an electrochemical process to create a hard, protective oxide coating on the surface. This coating makes the aluminum even more resistant to corrosion, wear, and abrasion. It also gives the aluminum a smooth, attractive finish that is easy to clean and maintain. Anodized Aluminum 6061, a specialized treatment of the versatile aluminum alloy, enhances its surface properties for many applications, including those in the pharmaceutical industry.
Pharmaceutical Uses: In pharmaceuticals, Anodized Aluminum 6061 is employed to manufacture components such as lab equipment, cabinets, and cleanroom fixtures. The anodizing process creates a protective oxide layer on the aluminum surface, providing superior corrosion resistance and improved durability. This makes it well-suited for pharmaceutical cleanrooms where maintaining a sterile environment is critical. The enhanced surface hardness of anodized Aluminum 6061 also ensures resistance to wear and abrasion, prolonging the lifespan of the equipment.
Beyond pharmaceuticals, this material finds applications in aerospace, electronics, and architectural projects, where its aesthetic appeal, corrosion resistance, and durability are highly valued.
Borosilicate Glass
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Borosilicate glass is a type of glass primarily composed of silica and boron trioxide, serving as the key glass-forming constituents. This glass is renowned for its exceptionally low coefficients of thermal expansion, approximately 3 × 10^-6 K^-1 at 20°C. This unique property renders borosilicate glass highly resistant to thermal shock, surpassing the capabilities of most other common types of glass. It can endure significant temperature differentials without fracturing, withstanding variations of around 165°C (300°F).Borosilicate glass finds diverse applications, including the construction of reagent bottles and flasks, as well as use in lighting, electronics, and cookware.
Numerous trade names are associated with borosilicate glass, such as Borosil, Duran, Pyrex, Glassco, Supertek, Suprax, Simax, Bellco, Marinex (in Brazil), BSA 60, BSC 51 (by NIPRO), Heatex, Endural, Schott, Refmex, Kimax, Gemstone Well, United Scientific, and MG (in India). The production of borosilicate glass involves the fusion of boric oxide, silica sand, soda ash, and alumina. Notably, this glass melts at a higher temperature than ordinary silicate glass, necessitating distinct techniques for industrial production.
Pharmaceutical Uses: In the pharmaceutical sector, borosilicate glass is commonly used for manufacturing laboratory glassware like beakers, test tubes, and flasks. Its high thermal shock resistance allows for precise heating and cooling, crucial for pharmaceutical research and experimentation. Moreover, borosilicate glass's resistance to chemicals ensures it remains inert and does not react with pharmaceutical substances, preserving the integrity of experiments and tests.
Beyond pharmaceuticals, this glass is also widely used in the production of high-quality kitchenware, lighting fixtures, and optical lenses due to its clarity and strength.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Borosilicate glass is a type of glass primarily composed of silica and boron trioxide, serving as the key glass-forming constituents. This glass is renowned for its exceptionally low coefficients of thermal expansion, approximately 3 × 10^-6 K^-1 at 20°C. This unique property renders borosilicate glass highly resistant to thermal shock, surpassing the capabilities of most other common types of glass. It can endure significant temperature differentials without fracturing, withstanding variations of around 165°C (300°F).Borosilicate glass finds diverse applications, including the construction of reagent bottles and flasks, as well as use in lighting, electronics, and cookware.
Numerous trade names are associated with borosilicate glass, such as Borosil, Duran, Pyrex, Glassco, Supertek, Suprax, Simax, Bellco, Marinex (in Brazil), BSA 60, BSC 51 (by NIPRO), Heatex, Endural, Schott, Refmex, Kimax, Gemstone Well, United Scientific, and MG (in India). The production of borosilicate glass involves the fusion of boric oxide, silica sand, soda ash, and alumina. Notably, this glass melts at a higher temperature than ordinary silicate glass, necessitating distinct techniques for industrial production.
Pharmaceutical Uses: In the pharmaceutical sector, borosilicate glass is commonly used for manufacturing laboratory glassware like beakers, test tubes, and flasks. Its high thermal shock resistance allows for precise heating and cooling, crucial for pharmaceutical research and experimentation. Moreover, borosilicate glass's resistance to chemicals ensures it remains inert and does not react with pharmaceutical substances, preserving the integrity of experiments and tests.
Beyond pharmaceuticals, this glass is also widely used in the production of high-quality kitchenware, lighting fixtures, and optical lenses due to its clarity and strength.
Ethylene Propylene Diene Monomer (EPDM)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: EPDM rubber, which stands for ethylene propylene diene monomer rubber, is a versatile synthetic rubber widely used in various applications. In the manufacturing of EPDM rubber, dienes such as ethylidene norbornene (ENB), dicyclopentadiene (DCPD), and vinyl norbornene (VNB) are employed.EPDM rubber is categorized as an M-Class rubber according to the ASTM standard D-1418. The M class consists of elastomers with a saturated polyethylene chain. EPDM is composed of three primary components: ethylene, propylene, and a diene comonomer. The presence of this diene comonomer allows for crosslinking through sulfur vulcanization, a process that enhances the material's properties and performance. It is valued for its exceptional weather resistance, durability, and versatility across various industries, including pharmaceuticals.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: EPDM rubber, which stands for ethylene propylene diene monomer rubber, is a versatile synthetic rubber widely used in various applications. In the manufacturing of EPDM rubber, dienes such as ethylidene norbornene (ENB), dicyclopentadiene (DCPD), and vinyl norbornene (VNB) are employed.EPDM rubber is categorized as an M-Class rubber according to the ASTM standard D-1418. The M class consists of elastomers with a saturated polyethylene chain. EPDM is composed of three primary components: ethylene, propylene, and a diene comonomer. The presence of this diene comonomer allows for crosslinking through sulfur vulcanization, a process that enhances the material's properties and performance. It is valued for its exceptional weather resistance, durability, and versatility across various industries, including pharmaceuticals.
Pharmaceutical Uses: In pharmaceutical applications, EPDM is primarily used for manufacturing seals, gaskets, and O-rings. Its resilience and resistance to a wide range of chemicals and extreme temperatures make it ideal for ensuring a secure and contamination-free environment for pharmaceutical processes. EPDM's biocompatibility and low extractable levels are crucial for maintaining product purity in pharmaceutical manufacturing.
Beyond pharmaceuticals, EPDM is extensively employed in construction, automotive, and HVAC industries, where its resistance to UV radiation, ozone, and harsh environmental conditions make it a preferred choice for gaskets, roofing, and insulation.
Beyond pharmaceuticals, EPDM is extensively employed in construction, automotive, and HVAC industries, where its resistance to UV radiation, ozone, and harsh environmental conditions make it a preferred choice for gaskets, roofing, and insulation.
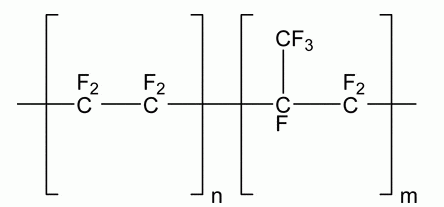
Fluorinated Ethylene Propylene (FEP)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Fluorinated ethylene propylene (FEP) is a copolymer formed by combining hexafluoropropylene and tetrafluoroethylene. FEP is a type of fluoropolymer that combines the excellent chemical resistance of PTFE (Polytetrafluoroethylene) with the flexibility and transparency of polyethylene. What sets FEP apart from polytetrafluoroethylene (PTFE) resins is its ability to be processed through conventional methods like injection molding and screw extrusion due to its melt-processable nature. FEP is created through a free-radical polymerization process involving mixtures of tetrafluoroethylene and hexafluoropropylene. This characteristic makes FEP a valuable material for various applications where melt-processing is required. This unique combination makes FEP a preferred material in various industries, including the pharmaceutical sector.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Fluorinated ethylene propylene (FEP) is a copolymer formed by combining hexafluoropropylene and tetrafluoroethylene. FEP is a type of fluoropolymer that combines the excellent chemical resistance of PTFE (Polytetrafluoroethylene) with the flexibility and transparency of polyethylene. What sets FEP apart from polytetrafluoroethylene (PTFE) resins is its ability to be processed through conventional methods like injection molding and screw extrusion due to its melt-processable nature. FEP is created through a free-radical polymerization process involving mixtures of tetrafluoroethylene and hexafluoropropylene. This characteristic makes FEP a valuable material for various applications where melt-processing is required. This unique combination makes FEP a preferred material in various industries, including the pharmaceutical sector.
Pharmaceutical Uses: In pharmaceuticals, FEP is utilized for its non-reactive nature and high purity, making it ideal for manufacturing components like tubing and seals used in the transportation and containment of sensitive drugs and chemicals. Its non-stick and low-friction properties also make FEP suitable for applications in lab equipment, ensuring smooth and consistent processes.
Additionally, FEP's resistance to heat and chemicals extends its use to industries such as electronics, automotive, and food processing, where it provides exceptional performance and reliability.
Additionally, FEP's resistance to heat and chemicals extends its use to industries such as electronics, automotive, and food processing, where it provides exceptional performance and reliability.
BRASS
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Brass is an alloy primarily composed of copper (Cu) and zinc (Zn), and the specific ratios of these elements can be adjusted to achieve varying colors and a wide range of mechanical, electrical, acoustic, and chemical properties. Typically, copper makes up the larger proportion of the alloy. This versatile material has been in use since ancient times and is classified as a substitutional alloy, wherein atoms of copper and zinc can interchange positions within the same crystal structure. Brass is known for its malleability, surpassing that of bronze or zinc.One of the remarkable characteristics of brass is its relatively low melting point, which falls within the range of 900 to 940°C (1,650 to 1,720°F), depending on the specific composition. This, along with its favorable flow properties, makes brass a relatively easy material for casting. By altering the proportions of copper and zinc, the properties of brass can be fine-tuned, resulting in both hard and soft brass variants. The density of brass typically ranges from 8.4 to 8.73 g/cm3 (0.303 to 0.315 lb/cu in). Importantly, nearly 90% of all brass alloys are recycled. Additionally, brass is not ferromagnetic, meaning it does not exhibit magnetic properties.
Pharmaceutical Uses: In pharmaceutical applications, brass is often utilized for manufacturing precision components and equipment, such as valves, fittings, and instrumentation. Its excellent machinability, corrosion resistance, and thermal conductivity are advantageous in pharmaceutical processing equipment, ensuring reliability and efficient operations. Additionally, brass's durability and ability to withstand chemical exposure make it suitable for applications involving pharmaceutical compounds and cleaning agents.
Beyond pharmaceuticals, this versatile alloy is employed in plumbing, electrical, and automotive industries, where its strength, malleability, and aesthetic appeal contribute to its widespread use.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Brass is an alloy primarily composed of copper (Cu) and zinc (Zn), and the specific ratios of these elements can be adjusted to achieve varying colors and a wide range of mechanical, electrical, acoustic, and chemical properties. Typically, copper makes up the larger proportion of the alloy. This versatile material has been in use since ancient times and is classified as a substitutional alloy, wherein atoms of copper and zinc can interchange positions within the same crystal structure. Brass is known for its malleability, surpassing that of bronze or zinc.One of the remarkable characteristics of brass is its relatively low melting point, which falls within the range of 900 to 940°C (1,650 to 1,720°F), depending on the specific composition. This, along with its favorable flow properties, makes brass a relatively easy material for casting. By altering the proportions of copper and zinc, the properties of brass can be fine-tuned, resulting in both hard and soft brass variants. The density of brass typically ranges from 8.4 to 8.73 g/cm3 (0.303 to 0.315 lb/cu in). Importantly, nearly 90% of all brass alloys are recycled. Additionally, brass is not ferromagnetic, meaning it does not exhibit magnetic properties.
Pharmaceutical Uses: In pharmaceutical applications, brass is often utilized for manufacturing precision components and equipment, such as valves, fittings, and instrumentation. Its excellent machinability, corrosion resistance, and thermal conductivity are advantageous in pharmaceutical processing equipment, ensuring reliability and efficient operations. Additionally, brass's durability and ability to withstand chemical exposure make it suitable for applications involving pharmaceutical compounds and cleaning agents.
Beyond pharmaceuticals, this versatile alloy is employed in plumbing, electrical, and automotive industries, where its strength, malleability, and aesthetic appeal contribute to its widespread use.
HASTELLOY
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Hastelloy is a term used to describe nickel-based alloys that have been specifically formulated to enhance their resistance to corrosion. This is primarily achieved by adding elements like molybdenum and chromium to the nickel base. There are numerous grades of Hastelloy, and each grade is tailored with a unique chemical composition to optimize specific properties for various applications.
Hastelloy alloys, which typically feature substantial amounts of molybdenum and chromium, are particularly well-suited for use in highly corrosive environments when compared to other specialty metals like Incoloy. The addition of molybdenum not only improves corrosion resistance but also enhances the overall workability of Hastelloy alloys. These alloys are ductile and can be readily fabricated and shaped to meet specific requirements.
The manufacturing of Hastelloy involves combining raw elements in a molten state, typically through smelting and alloying processes. Different grades may contain varying proportions of key elements. For example, a Hastelloy alloy may consist of between 1% and 25% chromium, 5% to 30% molybdenum, and 0% to 30% iron, with the rest being nickel. Depending on the specific grade and desired properties, additional additives like carbon, tungsten, vanadium, and titanium may also be incorporated. Once the alloying process is complete, the resulting material can be cast into various forms for further manufacturing, making it a valuable choice for applications where corrosion resistance and robust performance are essential.
Pharmaceutical Uses: In pharmaceutical applications, Hastelloy alloys are employed for their remarkable corrosion resistance, making them ideal for manufacturing critical components like reactors, vessels, and piping systems used in the production of pharmaceutical chemicals and compounds. The ability of Hastelloy to withstand highly corrosive substances and maintain product purity is crucial in ensuring the quality and safety of pharmaceutical products.
Beyond pharmaceuticals, Hastelloy alloys are also indispensable in industries such as chemical processing, aerospace, and oil and gas, where their superior performance in hostile environments is of paramount importance.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Hastelloy is a term used to describe nickel-based alloys that have been specifically formulated to enhance their resistance to corrosion. This is primarily achieved by adding elements like molybdenum and chromium to the nickel base. There are numerous grades of Hastelloy, and each grade is tailored with a unique chemical composition to optimize specific properties for various applications.
Hastelloy alloys, which typically feature substantial amounts of molybdenum and chromium, are particularly well-suited for use in highly corrosive environments when compared to other specialty metals like Incoloy. The addition of molybdenum not only improves corrosion resistance but also enhances the overall workability of Hastelloy alloys. These alloys are ductile and can be readily fabricated and shaped to meet specific requirements.
The manufacturing of Hastelloy involves combining raw elements in a molten state, typically through smelting and alloying processes. Different grades may contain varying proportions of key elements. For example, a Hastelloy alloy may consist of between 1% and 25% chromium, 5% to 30% molybdenum, and 0% to 30% iron, with the rest being nickel. Depending on the specific grade and desired properties, additional additives like carbon, tungsten, vanadium, and titanium may also be incorporated. Once the alloying process is complete, the resulting material can be cast into various forms for further manufacturing, making it a valuable choice for applications where corrosion resistance and robust performance are essential.
Pharmaceutical Uses: In pharmaceutical applications, Hastelloy alloys are employed for their remarkable corrosion resistance, making them ideal for manufacturing critical components like reactors, vessels, and piping systems used in the production of pharmaceutical chemicals and compounds. The ability of Hastelloy to withstand highly corrosive substances and maintain product purity is crucial in ensuring the quality and safety of pharmaceutical products.
Beyond pharmaceuticals, Hastelloy alloys are also indispensable in industries such as chemical processing, aerospace, and oil and gas, where their superior performance in hostile environments is of paramount importance.
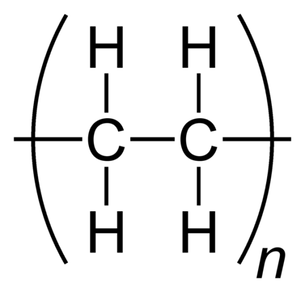
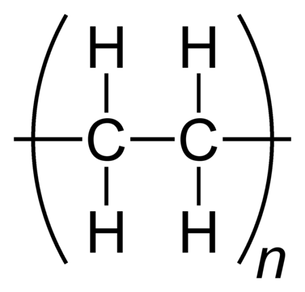
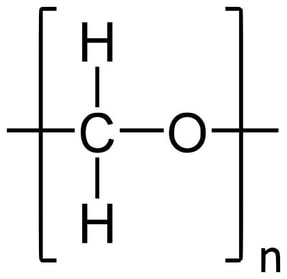
High Density Polyethylene (HDPE)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: High-density polyethylene (HDPE), also known as polyethylene high-density (PEHD), is a thermoplastic polymer formed from the monomer ethylene. Renowned for its exceptional strength-to-density ratio, HDPE finds application in the manufacturing of plastic bottles, corrosion-resistant piping, geomembranes, and plastic lumber. HDPE is notable for its recyclability, and it is identified by the resin identification code "2."The density of HDPE typically falls within the range of 930 to 970 kg/m3. One of its defining characteristics is its limited branching, which results in stronger intermolecular forces and higher tensile strength compared to low-density polyethylene (LDPE). Specifically, HDPE exhibits a tensile strength of 38 MPa, as opposed to LDPE's 21 MPa. This discrepancy in strength surpasses the difference in density, giving HDPE a superior specific strength. Moreover, HDPE is harder and more opaque, and it can endure relatively higher temperatures, withstanding short-term exposure to temperatures as high as 120°C (248°F).
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: High-density polyethylene (HDPE), also known as polyethylene high-density (PEHD), is a thermoplastic polymer formed from the monomer ethylene. Renowned for its exceptional strength-to-density ratio, HDPE finds application in the manufacturing of plastic bottles, corrosion-resistant piping, geomembranes, and plastic lumber. HDPE is notable for its recyclability, and it is identified by the resin identification code "2."The density of HDPE typically falls within the range of 930 to 970 kg/m3. One of its defining characteristics is its limited branching, which results in stronger intermolecular forces and higher tensile strength compared to low-density polyethylene (LDPE). Specifically, HDPE exhibits a tensile strength of 38 MPa, as opposed to LDPE's 21 MPa. This discrepancy in strength surpasses the difference in density, giving HDPE a superior specific strength. Moreover, HDPE is harder and more opaque, and it can endure relatively higher temperatures, withstanding short-term exposure to temperatures as high as 120°C (248°F).
Pharmaceutical Uses: In the pharmaceutical industry, HDPE is extensively used for manufacturing bottles, containers, and closures for liquid and solid medications. Its robust and inert nature ensures that the pharmaceutical products remain safe and uncontaminated. Additionally, HDPE's ability to withstand moisture and moisture vapor transmission makes it suitable for protecting sensitive drugs from environmental factors.
Beyond pharmaceuticals, HDPE is also used in industries such as food packaging, construction, and healthcare for its reliability and versatility.
Beyond pharmaceuticals, HDPE is also used in industries such as food packaging, construction, and healthcare for its reliability and versatility.
Low Density Polyethylene (LDPE)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Low-density polyethylene (LDPE) is a thermoplastic polymer derived from the monomer ethylene, making it the earliest variant of polyethylene. LDPE is characterized by a specific density range of 917–930 kg/m3. At room temperature, LDPE exhibits minimal reactivity, except when exposed to potent oxidizers. Certain solvents may cause it to swell. LDPE is capable of withstanding continuous temperatures of up to 65°C (149°F) and short-term exposure to temperatures of 90°C (194°F).This versatile material is available in both translucent and opaque forms, and it is known for its remarkable flexibility and toughness. LDPE differs from high-density polyethylene (HDPE) due to a higher degree of branching (occurring in approximately 2% of carbon atoms). This branching results in weaker intermolecular forces, lower tensile strength, and greater resilience. Additionally, the presence of side branches leads to less densely packed and less crystalline molecular structures, contributing to LDPE's lower density.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Low-density polyethylene (LDPE) is a thermoplastic polymer derived from the monomer ethylene, making it the earliest variant of polyethylene. LDPE is characterized by a specific density range of 917–930 kg/m3. At room temperature, LDPE exhibits minimal reactivity, except when exposed to potent oxidizers. Certain solvents may cause it to swell. LDPE is capable of withstanding continuous temperatures of up to 65°C (149°F) and short-term exposure to temperatures of 90°C (194°F).This versatile material is available in both translucent and opaque forms, and it is known for its remarkable flexibility and toughness. LDPE differs from high-density polyethylene (HDPE) due to a higher degree of branching (occurring in approximately 2% of carbon atoms). This branching results in weaker intermolecular forces, lower tensile strength, and greater resilience. Additionally, the presence of side branches leads to less densely packed and less crystalline molecular structures, contributing to LDPE's lower density.
Pharmaceutical Uses: In pharmaceuticals, LDPE is commonly employed for manufacturing flexible and squeezable bottles, dropper bottles, and film packaging for medications. Its flexibility allows for easy dispensing of liquid and semi-liquid pharmaceutical products, while its chemical resistance ensures product integrity. LDPE is particularly valuable for packaging solutions where precise dosing and contamination prevention are crucial.
Beyond pharmaceuticals, LDPE is utilized in various industries, including food packaging, agriculture, and construction, due to its versatility and ability to withstand harsh environmental conditions.
Beyond pharmaceuticals, LDPE is utilized in various industries, including food packaging, agriculture, and construction, due to its versatility and ability to withstand harsh environmental conditions.
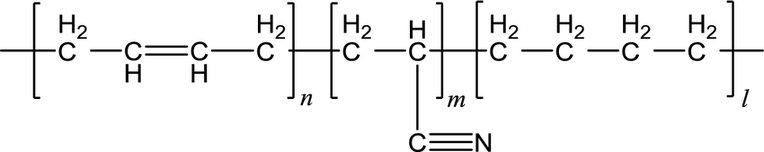
Nitrile Butadiene Rubber (NBR)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Nitrile butadiene rubber, often referred to as NBR, is a synthetic rubber that originates from the combination of acrylonitrile (ACN) and butadiene. It's known by various trade names, including Perbunan, Nipol, Krynac, and Europrene. What distinguishes NBR is its exceptional resistance to a wide range of substances, such as oil, fuel, and various chemicals.The synthesis of NBR involves several key components. These include emulsifier (often in the form of soap), acrylonitrile, butadiene, radical-generating activators, and a catalyst. These elements are carefully combined in polymerization vessels, resulting in the creation of nitrile butadiene rubber.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Nitrile butadiene rubber, often referred to as NBR, is a synthetic rubber that originates from the combination of acrylonitrile (ACN) and butadiene. It's known by various trade names, including Perbunan, Nipol, Krynac, and Europrene. What distinguishes NBR is its exceptional resistance to a wide range of substances, such as oil, fuel, and various chemicals.The synthesis of NBR involves several key components. These include emulsifier (often in the form of soap), acrylonitrile, butadiene, radical-generating activators, and a catalyst. These elements are carefully combined in polymerization vessels, resulting in the creation of nitrile butadiene rubber.
Pharmaceutical Uses: NBR's unique combination of properties, such as resistance to chemicals, heat, and abrasion, makes it ideal for manufacturing pharmaceutical-grade seals, gaskets, and O-rings. These components play a critical role in maintaining the integrity of pharmaceutical processing equipment and ensuring the containment of sensitive pharmaceutical products. NBR is favored for its ability to maintain its elasticity and sealing capabilities even when exposed to a range of chemicals and temperatures, ensuring the safety and purity of pharmaceutical products.
Beyond the pharmaceutical industry, NBR is extensively used in automotive, oil and gas, and industrial applications where sealing performance is essential.
Beyond the pharmaceutical industry, NBR is extensively used in automotive, oil and gas, and industrial applications where sealing performance is essential.
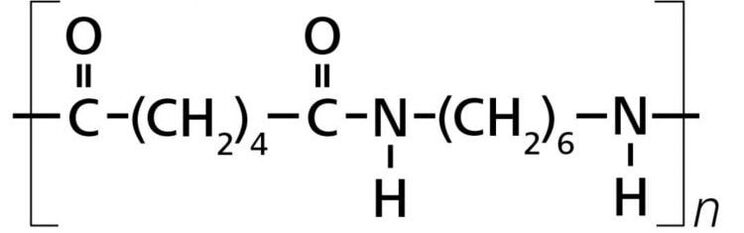
NYLON 6/6
NOTE: Use the link above in the coupon name to go to the product page to purchase
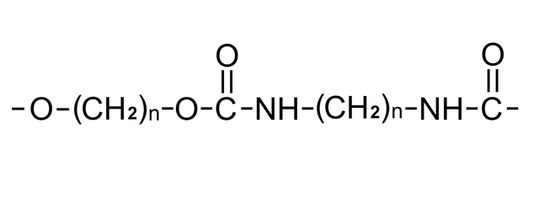
Description: Nylon 66, a member of the polyamide or nylon family, along with nylon 6, stands out as one of the most widely used materials in the textile and plastic industries. The name "Nylon 66" arises from its composition of two monomers, each featuring 6 carbon atoms: hexamethylenediamine and adipic acid. What makes Nylon 66 particularly appealing is not only its impressive physical properties but also the cost-effectiveness of its precursors. This versatile material is synthesized through the process of polycondensation, wherein hexamethylenediamine and adipic acid are combined to create Nylon 66.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Nylon 66, a member of the polyamide or nylon family, along with nylon 6, stands out as one of the most widely used materials in the textile and plastic industries. The name "Nylon 66" arises from its composition of two monomers, each featuring 6 carbon atoms: hexamethylenediamine and adipic acid. What makes Nylon 66 particularly appealing is not only its impressive physical properties but also the cost-effectiveness of its precursors. This versatile material is synthesized through the process of polycondensation, wherein hexamethylenediamine and adipic acid are combined to create Nylon 66.
Pharmaceutical Uses: In pharmaceuticals, Nylon 6/6 is commonly utilized for manufacturing precision parts and components for pharmaceutical processing equipment. Its high mechanical strength and resistance to chemicals make it an ideal choice for producing gears, bushings, and other mechanical components that are essential for pharmaceutical machinery. Additionally, Nylon 6/6's ability to maintain its properties even in humid environments makes it suitable for applications where moisture resistance is required.
Beyond the pharmaceutical industry, Nylon 6/6 finds use in automotive, electrical, and industrial applications, where its combination of strength and versatility is highly valued.
Beyond the pharmaceutical industry, Nylon 6/6 finds use in automotive, electrical, and industrial applications, where its combination of strength and versatility is highly valued.
NYLON 6/12
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Nylon 12, a nylon polymer with the chemical formula [(CH2)11C(O)NH]n, derives its name from its composition. It is synthesized from monomers of ω-aminolauric acid or laurolactam, both of which contain 12 carbon atoms. This 12-carbon structure gives rise to the name 'Nylon 12'.
The production of Nylon 12 can occur via two distinct routes. The first method involves polycondensation of ω-aminolauric acid, which is a bifunctional monomer characterized by one amine and one carboxylic acid group. The second route employs ring-opening polymerization of laurolactam, a process conducted at temperatures ranging from 260 to 300 degrees Celsius.
It is a versatile thermoplastic polymer renowned for its exceptional balance of properties. Composed of long chains of carbon, oxygen, and nitrogen atoms, this material exhibits high strength, low moisture absorption, and excellent chemical resistance.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Nylon 12, a nylon polymer with the chemical formula [(CH2)11C(O)NH]n, derives its name from its composition. It is synthesized from monomers of ω-aminolauric acid or laurolactam, both of which contain 12 carbon atoms. This 12-carbon structure gives rise to the name 'Nylon 12'.
The production of Nylon 12 can occur via two distinct routes. The first method involves polycondensation of ω-aminolauric acid, which is a bifunctional monomer characterized by one amine and one carboxylic acid group. The second route employs ring-opening polymerization of laurolactam, a process conducted at temperatures ranging from 260 to 300 degrees Celsius.
It is a versatile thermoplastic polymer renowned for its exceptional balance of properties. Composed of long chains of carbon, oxygen, and nitrogen atoms, this material exhibits high strength, low moisture absorption, and excellent chemical resistance.
Pharmaceutical Uses: In the pharmaceutical industry, Nylon 6/12 finds crucial applications due to its compatibility with various chemicals and its low moisture absorption, which ensures the integrity of pharmaceutical products. It is used for manufacturing precision components, such as tubing, hoses, and seals, that play a vital role in pharmaceutical processing equipment.
Beyond pharmaceuticals, Nylon 6/12's durability and resistance to oils and chemicals make it valuable in the automotive, aerospace, and oil and gas industries, where reliability and performance are paramount.
Beyond pharmaceuticals, Nylon 6/12's durability and resistance to oils and chemicals make it valuable in the automotive, aerospace, and oil and gas industries, where reliability and performance are paramount.
Polyether Ether Ketone (PEEK)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polyether ether ketone (PEEK) is an organic thermoplastic polymer that falls within the polyaryletherketone (PAEK) family. This colorless material is extensively employed in engineering applications. PEEK polymer emerged from its initial development in November 1978 and is synthesized through step-growth polymerization, achieved by dialkylating bisphenolate salts. PEEK is classified as a semicrystalline thermoplastic, known for its exceptional mechanical and chemical resistance, attributes that remain effective even at elevated temperatures. It's worth noting that the processing conditions applied during the molding of PEEK can influence its crystallinity, thereby impacting its mechanical properties.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polyether ether ketone (PEEK) is an organic thermoplastic polymer that falls within the polyaryletherketone (PAEK) family. This colorless material is extensively employed in engineering applications. PEEK polymer emerged from its initial development in November 1978 and is synthesized through step-growth polymerization, achieved by dialkylating bisphenolate salts. PEEK is classified as a semicrystalline thermoplastic, known for its exceptional mechanical and chemical resistance, attributes that remain effective even at elevated temperatures. It's worth noting that the processing conditions applied during the molding of PEEK can influence its crystallinity, thereby impacting its mechanical properties.
Pharmaceutical Uses: In pharmaceuticals, PEEK is employed for manufacturing components like seals, connectors, and fittings used in high-temperature and chemically aggressive environments. Its resistance to a wide range of chemicals, including sterilization agents, ensures product purity and integrity. Furthermore, PEEK's ability to maintain mechanical properties at elevated temperatures makes it suitable for pharmaceutical processing equipment, contributing to the industry's safety and efficiency.
Beyond pharmaceuticals, PEEK is integral in aerospace, automotive, and medical device industries, where its combination of high-performance characteristics meets stringent requirements.
Beyond pharmaceuticals, PEEK is integral in aerospace, automotive, and medical device industries, where its combination of high-performance characteristics meets stringent requirements.
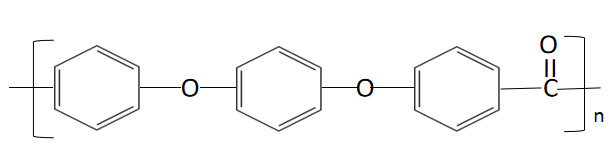
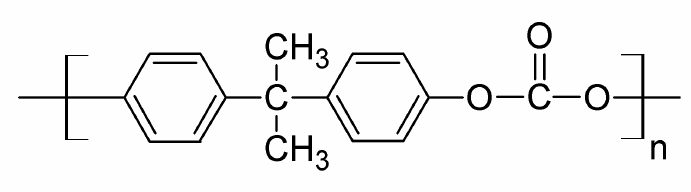
POLYCARBONATE (LEXAN)
NOTE: Use the link above in the coupon name to go to the product page to purchase
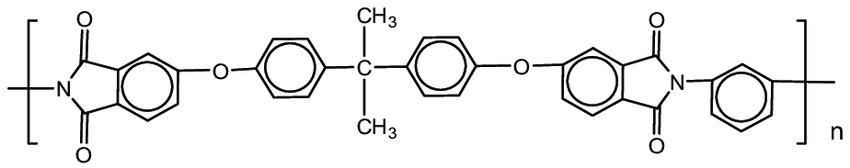
Description: Polycarbonates, often abbreviated as PC, belong to a category of thermoplastic polymers characterized by the presence of carbonate groups within their chemical structures. These engineering-grade polycarbonates exhibit remarkable strength and toughness, with select grades boasting optical transparency. Their versatile nature allows for ease of manipulation, molding, and thermoforming, making them highly adaptable for a wide range of applications. Notably, polycarbonates are designated as "Other," with a resin identification code (RIC) as they lack a distinct RIC of their own. It's important to note that products crafted from polycarbonate may include the precursor monomer known as bisphenol A (BPA).
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polycarbonates, often abbreviated as PC, belong to a category of thermoplastic polymers characterized by the presence of carbonate groups within their chemical structures. These engineering-grade polycarbonates exhibit remarkable strength and toughness, with select grades boasting optical transparency. Their versatile nature allows for ease of manipulation, molding, and thermoforming, making them highly adaptable for a wide range of applications. Notably, polycarbonates are designated as "Other," with a resin identification code (RIC) as they lack a distinct RIC of their own. It's important to note that products crafted from polycarbonate may include the precursor monomer known as bisphenol A (BPA).
Pharmaceutical Uses: In pharmaceutical applications, it is used to manufacture high-quality laboratory equipment like safety goggles, face shields, and transparent barriers. Its outstanding impact resistance ensures the protection and safety of personnel working with chemicals and biological substances. Additionally, polycarbonate's optical clarity makes it suitable for viewports in pharmaceutical cleanrooms, ensuring visibility while maintaining a sterile environment.
Beyond the pharmaceutical industry, polycarbonate is employed in automotive, electronics, and construction due to its excellent mechanical properties and resistance to extreme temperatures and UV radiation, making it a versatile and reliable material.
Beyond the pharmaceutical industry, polycarbonate is employed in automotive, electronics, and construction due to its excellent mechanical properties and resistance to extreme temperatures and UV radiation, making it a versatile and reliable material.
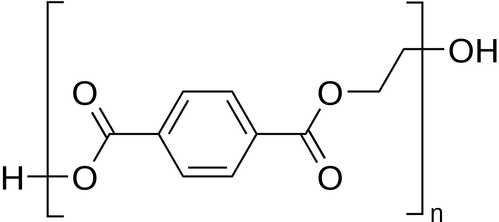
Polyethylene Terephthalate Glycol (PETG)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polyethylene terephthalate glycol, known as PETG or PET-G, is a versatile thermoplastic polyester renowned for its chemical resistance, durability, and formability, making it suitable for various manufacturing applications. PETG is derived from PET (Polyethylene terephthalate) by adding glycol at a molecular level, which imparts different chemical properties. Although PETG shares the same monomers as PET, it exhibits greater strength and durability, enhanced impact resistance, and better performance at higher temperatures. Due to its low forming temperatures, PETG is easily molded through vacuum and pressure forming or heat bending, making it a popular choice for consumer and commercial applications. These properties also make it a widely used material in 3D printing and other heat-forming processes. PETG is well-suited for techniques like bending, die cutting, and routing.
The modification of PET with glycol to create PETG involves replacing ethylene glycol in the molecular chain with a larger monomer, cyclohexane dimethanol. This alteration prevents crystallization, which is commonly associated with PET. As a result, PETG offers improved heat resistance, reduced crystallization, and a lower melting point.
Key properties of polyethylene terephthalate glycol (PETG) include hardness, chemical and impact resistance, transparency, and ductility. It is an easily extruded material with good thermal stability and is particularly compatible with food-related applications. PETG is highly suitable for 3D printing, with an extrusion temperature typically falling between 220°C and 260°C and a recommended print speed of 40-60mm/s. Its versatility and favorable properties have made PETG a popular choice in various industrial and manufacturing applications.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polyethylene terephthalate glycol, known as PETG or PET-G, is a versatile thermoplastic polyester renowned for its chemical resistance, durability, and formability, making it suitable for various manufacturing applications. PETG is derived from PET (Polyethylene terephthalate) by adding glycol at a molecular level, which imparts different chemical properties. Although PETG shares the same monomers as PET, it exhibits greater strength and durability, enhanced impact resistance, and better performance at higher temperatures. Due to its low forming temperatures, PETG is easily molded through vacuum and pressure forming or heat bending, making it a popular choice for consumer and commercial applications. These properties also make it a widely used material in 3D printing and other heat-forming processes. PETG is well-suited for techniques like bending, die cutting, and routing.
The modification of PET with glycol to create PETG involves replacing ethylene glycol in the molecular chain with a larger monomer, cyclohexane dimethanol. This alteration prevents crystallization, which is commonly associated with PET. As a result, PETG offers improved heat resistance, reduced crystallization, and a lower melting point.
Key properties of polyethylene terephthalate glycol (PETG) include hardness, chemical and impact resistance, transparency, and ductility. It is an easily extruded material with good thermal stability and is particularly compatible with food-related applications. PETG is highly suitable for 3D printing, with an extrusion temperature typically falling between 220°C and 260°C and a recommended print speed of 40-60mm/s. Its versatility and favorable properties have made PETG a popular choice in various industrial and manufacturing applications.
Pharmaceutical Uses: In pharmaceuticals, PETG is favored for its excellent clarity and chemical resistance, making it an ideal material for packaging containers like bottles, jars, and blister packs. These PETG containers provide a clear view of pharmaceutical products while ensuring the protection and integrity of sensitive medications. Additionally, PETG's ease of sterilization and compatibility with aseptic processes make it valuable in pharmaceutical cleanroom environments.
Beyond the pharmaceutical sector, PETG is commonly used in food packaging, medical devices, and signage due to its excellent balance of properties, making it a versatile choice for a wide range of applications.
Beyond the pharmaceutical sector, PETG is commonly used in food packaging, medical devices, and signage due to its excellent balance of properties, making it a versatile choice for a wide range of applications.
Polyoxymethylene Copolymer (POM-C)
NOTE: Use the link above in the coupon name to go to the product page to purchase
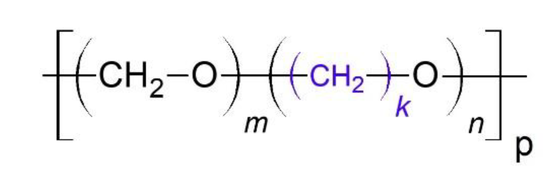
Description: Polyoxymethylene (POM), which is also known by names such as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic specifically designed for precision parts that require high stiffness, low friction, and excellent dimensional stability. Various chemical companies produce POM with slightly different formulas, and it's sold under different trade names like Delrin, Kocetal, Ultraform, Celcon, Ramtal, Duracon, Kepital, Polypenco, Tenac, and Hostaform.
POM is notable for its high strength, hardness, and rigidity, even at low temperatures down to -40°C. It is inherently opaque and white due to its high crystalline composition, but it can be manufactured in a range of colors. The density of POM typically falls within the range of 1.410 to 1.420 g/cm3.
Polyoxymethylene copolymer, a variant of POM, replaces approximately 1-1.5% of the -CH2O- groups with -CH2CH2O-. The production of polyoxymethylene copolymer usually involves the conversion of formaldehyde to trioxane. Stable polymer is formed through melt-compounding, which includes the addition of thermal and oxidative stabilizers, optional lubricants, and various fillers as needed for specific applications.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polyoxymethylene (POM), which is also known by names such as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic specifically designed for precision parts that require high stiffness, low friction, and excellent dimensional stability. Various chemical companies produce POM with slightly different formulas, and it's sold under different trade names like Delrin, Kocetal, Ultraform, Celcon, Ramtal, Duracon, Kepital, Polypenco, Tenac, and Hostaform.
POM is notable for its high strength, hardness, and rigidity, even at low temperatures down to -40°C. It is inherently opaque and white due to its high crystalline composition, but it can be manufactured in a range of colors. The density of POM typically falls within the range of 1.410 to 1.420 g/cm3.
Polyoxymethylene copolymer, a variant of POM, replaces approximately 1-1.5% of the -CH2O- groups with -CH2CH2O-. The production of polyoxymethylene copolymer usually involves the conversion of formaldehyde to trioxane. Stable polymer is formed through melt-compounding, which includes the addition of thermal and oxidative stabilizers, optional lubricants, and various fillers as needed for specific applications.
Pharmaceutical Uses: In pharmaceutical settings, POM-C is commonly employed for manufacturing components such as gears, valves, and fittings used in pharmaceutical processing equipment. Its low friction and high wear resistance ensure smooth and efficient operations, contributing to the pharmaceutical industry's quality and productivity. Moreover, POM-C's resistance to moisture and chemicals makes it suitable for applications requiring contact with pharmaceuticals and cleaning agents.
Beyond pharmaceuticals, POM-C is also used in the automotive, electronics, and consumer goods industries where its outstanding mechanical properties provide durability and performance.
Beyond pharmaceuticals, POM-C is also used in the automotive, electronics, and consumer goods industries where its outstanding mechanical properties provide durability and performance.
Polyoxymethylene Homopolymer (POM-H)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polyoxymethylene (POM) is an engineering thermoplastic used in precision parts that demand high stiffness, low friction, and excellent dimensional stability. Like many other synthetic polymers, it is manufactured by various chemical firms with slight variations in the formula. POM is known for its exceptional properties, including high strength, hardness, rigidity to -40°C, intrinsic opaque white color due to its high crystalline composition, and the ability to be produced in a variety of colors. These characteristics make POM a valuable material for precision components in various industries.The production of polyoxymethylene homopolymer involves a process where anhydrous (i.e., water-free) formaldehyde is generated. The primary method for achieving this is as follows:Reaction of Aqueous Formaldehyde with Alcohol: Aqueous formaldehyde is reacted with an alcohol to create a compound known as hemiformal. This process allows for the generation of anhydrous formaldehyde, which is a crucial component in the production of polyoxymethylene homopolymer. The formaldehyde is then used in polymerization reactions to create the homopolymer material.
POM-H is employed across various industries, including pharmaceuticals and for applications that require precision and reliability.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polyoxymethylene (POM) is an engineering thermoplastic used in precision parts that demand high stiffness, low friction, and excellent dimensional stability. Like many other synthetic polymers, it is manufactured by various chemical firms with slight variations in the formula. POM is known for its exceptional properties, including high strength, hardness, rigidity to -40°C, intrinsic opaque white color due to its high crystalline composition, and the ability to be produced in a variety of colors. These characteristics make POM a valuable material for precision components in various industries.The production of polyoxymethylene homopolymer involves a process where anhydrous (i.e., water-free) formaldehyde is generated. The primary method for achieving this is as follows:Reaction of Aqueous Formaldehyde with Alcohol: Aqueous formaldehyde is reacted with an alcohol to create a compound known as hemiformal. This process allows for the generation of anhydrous formaldehyde, which is a crucial component in the production of polyoxymethylene homopolymer. The formaldehyde is then used in polymerization reactions to create the homopolymer material.
POM-H is employed across various industries, including pharmaceuticals and for applications that require precision and reliability.
Pharmaceutical Uses: In the pharmaceutical sector, POM-H is frequently used for manufacturing components like gears, conveyor belts, and valve components used in pharmaceutical processing equipment. Its exceptional mechanical strength, low friction, and high wear resistance ensure smooth and efficient operations, contributing to the pharmaceutical industry's safety and productivity. Additionally, POM-H's resistance to moisture and chemicals makes it suitable for applications involving contact with pharmaceuticals and cleaning agents.
Beyond pharmaceuticals, POM-H finds use in the automotive, aerospace, and consumer goods industries where its outstanding mechanical properties provide durability and performance.
Beyond pharmaceuticals, POM-H finds use in the automotive, aerospace, and consumer goods industries where its outstanding mechanical properties provide durability and performance.
POLYPROPYLENE (PP)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polypropylene (PP), also known as polypropene, is a versatile thermoplastic polymer used in a wide range of applications. It is produced through chain-growth polymerization from the monomer propylene. Polypropylene is classified as a member of the polyolefin group and is characterized as partially crystalline and non-polar. Its properties are similar to polyethylene but exhibit some distinct characteristics. Polypropylene is slightly harder and more heat-resistant. It has a white color, is mechanically rugged, and offers high chemical resistance.
In many aspects, polypropylene is similar to polyethylene, especially concerning its solution behavior and electrical properties. The addition of a methyl group to its structure improves its mechanical properties and thermal resistance, although it can lead to a decrease in chemical resistance.
The specific properties of polypropylene depend on factors such as molecular weight, molecular weight distribution, crystallinity, the type and proportion of comonomer (if used), and isotacticity. The density of polypropylene typically falls within the range of 0.895 to 0.93 g/cm3, making it one of the plastics with the lowest density. Purely isotactic polypropylene has a melting point of 171°C (340°F), while commercial isotactic PP exhibits a melting point ranging from 160 to 166°C (320 to 331°F), depending on factors like atactic material and crystallinity. Polypropylene demonstrates resistance to fats and is generally impervious to most organic solvents at room temperature, except for strong oxidants. These qualities make it a widely used commodity plastic in various industries.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polypropylene (PP), also known as polypropene, is a versatile thermoplastic polymer used in a wide range of applications. It is produced through chain-growth polymerization from the monomer propylene. Polypropylene is classified as a member of the polyolefin group and is characterized as partially crystalline and non-polar. Its properties are similar to polyethylene but exhibit some distinct characteristics. Polypropylene is slightly harder and more heat-resistant. It has a white color, is mechanically rugged, and offers high chemical resistance.
In many aspects, polypropylene is similar to polyethylene, especially concerning its solution behavior and electrical properties. The addition of a methyl group to its structure improves its mechanical properties and thermal resistance, although it can lead to a decrease in chemical resistance.
The specific properties of polypropylene depend on factors such as molecular weight, molecular weight distribution, crystallinity, the type and proportion of comonomer (if used), and isotacticity. The density of polypropylene typically falls within the range of 0.895 to 0.93 g/cm3, making it one of the plastics with the lowest density. Purely isotactic polypropylene has a melting point of 171°C (340°F), while commercial isotactic PP exhibits a melting point ranging from 160 to 166°C (320 to 331°F), depending on factors like atactic material and crystallinity. Polypropylene demonstrates resistance to fats and is generally impervious to most organic solvents at room temperature, except for strong oxidants. These qualities make it a widely used commodity plastic in various industries.
Pharmaceutical Uses: In pharmaceuticals, it plays a pivotal role by serving as the material of choice for crafting essential laboratory equipment such as bottles, vials, and containers, thanks to its exceptional resistance to chemicals. This chemical resilience ensures the secure storage and transport of pharmaceutical products while preserving their integrity. Furthermore, PP's ability to endure moisture and its compatibility with sterilization processes make it an ideal candidate for maintaining pristine conditions in pharmaceutical cleanrooms.
Beyond the pharmaceutical realm, PP finds extensive application in sectors spanning from food packaging to automotive components and medical devices. Its unique combination of attributes, including lightweight construction, toughness, and recyclability, makes it an extraordinarily versatile and sustainable material.
Beyond the pharmaceutical realm, PP finds extensive application in sectors spanning from food packaging to automotive components and medical devices. Its unique combination of attributes, including lightweight construction, toughness, and recyclability, makes it an extraordinarily versatile and sustainable material.
POLYSTYRENE
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polystyrene is a versatile thermoplastic polymer known for its rigid and lightweight properties. Polystyrene (PS) is a synthetic polymer derived from monomers of the aromatic hydrocarbon styrene. It can exist in both solid and foamed forms. General-purpose polystyrene is known for being clear, hard, and brittle. It is also relatively inexpensive per unit weight. However, it has limitations, including being a poor barrier to air and water vapor and having a relatively low melting point.
Polystyrene is among the most widely used plastics globally, with production volumes reaching several million tonnes annually. It is naturally transparent but can be colored using colorants. In chemical terms, polystyrene is a long-chain hydrocarbon where alternating carbon centers are attached to phenyl groups (derivatives of benzene). Its chemical formula is (C8H8)n, consisting of the elements carbon and hydrogen. Polystyrene is an addition polymer formed when styrene monomers undergo polymerization, resulting in the interconnection of these monomers.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polystyrene is a versatile thermoplastic polymer known for its rigid and lightweight properties. Polystyrene (PS) is a synthetic polymer derived from monomers of the aromatic hydrocarbon styrene. It can exist in both solid and foamed forms. General-purpose polystyrene is known for being clear, hard, and brittle. It is also relatively inexpensive per unit weight. However, it has limitations, including being a poor barrier to air and water vapor and having a relatively low melting point.
Polystyrene is among the most widely used plastics globally, with production volumes reaching several million tonnes annually. It is naturally transparent but can be colored using colorants. In chemical terms, polystyrene is a long-chain hydrocarbon where alternating carbon centers are attached to phenyl groups (derivatives of benzene). Its chemical formula is (C8H8)n, consisting of the elements carbon and hydrogen. Polystyrene is an addition polymer formed when styrene monomers undergo polymerization, resulting in the interconnection of these monomers.
Pharmaceutical Uses: In the pharmaceutical sector, polystyrene finds prominent use in the manufacturing of labware such as Petri dishes, test tubes, and sample containers. These items are favored for their transparency, allowing for easy observation and analysis of pharmaceutical samples. Polystyrene's smooth surface also facilitates the growth of cell cultures, making it invaluable in research and drug development.
Beyond pharmaceuticals, polystyrene is utilized extensively in packaging, construction, and the food industry, where its insulating properties contribute to its popularity in disposable cups and food containers.
Beyond pharmaceuticals, polystyrene is utilized extensively in packaging, construction, and the food industry, where its insulating properties contribute to its popularity in disposable cups and food containers.
Polytetrafluoroethylene (PTFE)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer derived from tetrafluoroethylene and belongs to the class of PFAS (per- and polyfluoroalkyl substances) with a wide range of applications. The most well-known brand name associated with PTFE-based compositions is Teflon. PTFE is a unique material with the following characteristics:
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer derived from tetrafluoroethylene and belongs to the class of PFAS (per- and polyfluoroalkyl substances) with a wide range of applications. The most well-known brand name associated with PTFE-based compositions is Teflon. PTFE is a unique material with the following characteristics:
- Fluorocarbon Solid: It is a high-molecular-weight polymer composed entirely of carbon and fluorine atoms.
- Hydrophobic Nature: PTFE is highly hydrophobic, meaning that water and water-containing substances do not adhere to it. This is because fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine.
- Low Friction: PTFE possesses one of the lowest coefficients of friction of any solid material, making it exceptionally slippery.
- Non-Stick Coating: It is widely used as a non-stick coating for cookware, ensuring that food doesn't stick to the surface.
- Chemical Resistance: Due to its non-reactive nature, often attributed to the strength of carbon-fluorine bonds, PTFE is employed in containers and pipework for handling reactive and corrosive chemicals.
Pharmaceutical Uses: In pharmaceuticals, PTFE is prized for its unparalleled chemical resistance, making it an ideal material for manufacturing components such as gaskets, seals, and liners that come into contact with aggressive chemicals and high-purity pharmaceutical products. Its non-stick properties also find utility in pharmaceutical processing equipment, ensuring efficient and clean operations. Furthermore, PTFE's resistance to extreme temperatures makes it a vital material for labware, ensuring consistent performance and the integrity of pharmaceutical processes.
Beyond the pharmaceutical industry, PTFE's exceptional properties make it invaluable in aerospace, electronics, and industrial applications, where reliability and durability are paramount.
Beyond the pharmaceutical industry, PTFE's exceptional properties make it invaluable in aerospace, electronics, and industrial applications, where reliability and durability are paramount.
Polyurethane (PU)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polyurethane (often abbreviated as PUR or PU) refers to a class of polymers made up of organic units connected by carbamate (urethane) links. Unlike other common polymers such as polyethylene and polystyrene, polyurethane is synthesized from a wide range of starting materials. This chemical versatility results in polyurethanes with diverse chemical structures, leading to a wide array of applications.These applications encompass rigid and flexible foams, coatings, adhesives, electrical potting compounds, and fibers like spandex and polyurethane laminate (PUL). The production of polyurethane typically involves the reaction of an isocyanate with a polyol.
One distinctive feature of polyurethanes is that they contain two types of monomers that polymerize sequentially, classifying them as alternating copolymers. Both the isocyanates and polyols used in polyurethane manufacturing have two or more functional groups per molecule, contributing to the unique properties and versatility of this class of polymers.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Polyurethane (often abbreviated as PUR or PU) refers to a class of polymers made up of organic units connected by carbamate (urethane) links. Unlike other common polymers such as polyethylene and polystyrene, polyurethane is synthesized from a wide range of starting materials. This chemical versatility results in polyurethanes with diverse chemical structures, leading to a wide array of applications.These applications encompass rigid and flexible foams, coatings, adhesives, electrical potting compounds, and fibers like spandex and polyurethane laminate (PUL). The production of polyurethane typically involves the reaction of an isocyanate with a polyol.
One distinctive feature of polyurethanes is that they contain two types of monomers that polymerize sequentially, classifying them as alternating copolymers. Both the isocyanates and polyols used in polyurethane manufacturing have two or more functional groups per molecule, contributing to the unique properties and versatility of this class of polymers.
Pharmaceutical Uses: Within pharmaceuticals, polyurethane is widely used for manufacturing flexible tubing, catheters, and medical devices due to its excellent biocompatibility and flexibility. These components are crucial for various medical applications, from drug delivery systems to minimally invasive procedures, where polyurethane ensures patient safety and comfort. Additionally, polyurethane's resistance to moisture and chemicals makes it suitable for pharmaceutical packaging, where it helps maintain product integrity during storage and transportation.
Beyond pharmaceuticals, polyurethane is extensively employed in industries such as automotive, construction, and textiles, where its versatility and durability are highly prized.
Beyond pharmaceuticals, polyurethane is extensively employed in industries such as automotive, construction, and textiles, where its versatility and durability are highly prized.
Silicone USP Grade VI
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Silicone, specifically designed to meet the stringent requirements of USP (United States Pharmacopeia) Class VI, stands as a highly specialized and biocompatible material extensively used in the pharmaceutical and medical industries. This silicone material has been meticulously crafted to adhere to the highest safety standards.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Silicone, specifically designed to meet the stringent requirements of USP (United States Pharmacopeia) Class VI, stands as a highly specialized and biocompatible material extensively used in the pharmaceutical and medical industries. This silicone material has been meticulously crafted to adhere to the highest safety standards.
Pharmaceutical Uses: In pharmaceutical applications, USP Class VI silicone plays an essential role in the production of medical devices, tubing, seals, and gaskets that come into direct contact with pharmaceutical products or patients. Its biocompatibility and non-reactive nature make it ideal for use in medical implants, drug delivery systems, and other critical healthcare applications. This silicone variant provides the utmost assurance of purity, ensuring the safety and reliability of pharmaceutical and medical products, a vital aspect of healthcare and patient well-being.
Polyetherimide (PEI, ULTEM)
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Ultem® (polyetherimide) is a semi-transparent high-strength plastic material known for its ability to function effectively in high-service-temperature environments. Ultem® exhibits remarkable resistance to hot water and steam, making it capable of withstanding repeated cycles in a steam autoclave.Polyetherimide (PEI) is an amorphous thermoplastic that appears amber-to-transparent. It shares characteristics with another related plastic, PEEK. When compared to PEEK, PEI is a more cost-effective option but comes with trade-offs such as lower impact strength and a narrower temperature range.
The glass transition temperature of PEI is 217 °C (422 °F). It has an amorphous density of 1.27 g/cm3 (0.046 lb/in³) at 25 °C. One of its limitations is its susceptibility to stress cracking when exposed to chlorinated solvents.
One of the standout features of polyetherimide is its capacity to resist high temperatures while maintaining stable electrical properties across a wide range of frequencies. This combination of properties makes Ultem® a valuable material in applications where both heat resistance and electrical stability are crucial.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Ultem® (polyetherimide) is a semi-transparent high-strength plastic material known for its ability to function effectively in high-service-temperature environments. Ultem® exhibits remarkable resistance to hot water and steam, making it capable of withstanding repeated cycles in a steam autoclave.Polyetherimide (PEI) is an amorphous thermoplastic that appears amber-to-transparent. It shares characteristics with another related plastic, PEEK. When compared to PEEK, PEI is a more cost-effective option but comes with trade-offs such as lower impact strength and a narrower temperature range.
The glass transition temperature of PEI is 217 °C (422 °F). It has an amorphous density of 1.27 g/cm3 (0.046 lb/in³) at 25 °C. One of its limitations is its susceptibility to stress cracking when exposed to chlorinated solvents.
One of the standout features of polyetherimide is its capacity to resist high temperatures while maintaining stable electrical properties across a wide range of frequencies. This combination of properties makes Ultem® a valuable material in applications where both heat resistance and electrical stability are crucial.
Pharmaceutical Uses: In pharmaceuticals, Ultem/PEI is employed for manufacturing components like autoclavable labware, filtration membranes, and medical devices due to its exceptional resistance to high temperatures and harsh chemicals. This material's ability to maintain its mechanical properties even under extreme conditions ensures the reliability and safety of pharmaceutical processes and products.
Beyond pharmaceuticals, Ultem/PEI finds extensive use in aerospace, electronics, and automotive industries, where its combination of properties, including lightweight construction and flame resistance, make it an indispensable choice for high-performance applications.
Beyond pharmaceuticals, Ultem/PEI finds extensive use in aerospace, electronics, and automotive industries, where its combination of properties, including lightweight construction and flame resistance, make it an indispensable choice for high-performance applications.
VITON
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Viton (FKM), or fluorocarbon-based fluoroelastomer, is a family of elastomeric materials characterized by ASTM International standard D1418 and ISO standard 1629. It is commonly referred to as fluorine rubber or fluoro-rubber, with "FKM" standing for Fluorine Kautschuk Material. All FKMs share vinylidene fluoride as a common monomer.
Fluoroelastomers are recognized for their exceptional performance in harsh conditions, offering high-temperature resistance (up to 500°F or 260°C) and resistance to aggressive fluids, surpassing many other elastomers. They also demonstrate remarkable stability when exposed to various chemicals and fluids, including oil, diesel, ethanol blends, and bodily fluids.
The effectiveness of fluoroelastomers in resisting aggressive chemicals depends on the specific base polymer and the compounding ingredients used in the manufacturing process, such as for producing o-rings. While some formulations are generally compatible with hydrocarbons, they may be incompatible with substances like ketones (e.g., acetone and methyl ethyl ketone), ester solvents (e.g., ethyl acetate), amines, and organic acids like acetic acid.
One distinguishing characteristic of fluoroelastomers is their high density, exceeding 1800 kg/m³, which is significantly higher than most other types of rubber. This density is one of the factors that set them apart from many other elastomeric materials.
NOTE: Use the link above in the coupon name to go to the product page to purchase
Description: Viton (FKM), or fluorocarbon-based fluoroelastomer, is a family of elastomeric materials characterized by ASTM International standard D1418 and ISO standard 1629. It is commonly referred to as fluorine rubber or fluoro-rubber, with "FKM" standing for Fluorine Kautschuk Material. All FKMs share vinylidene fluoride as a common monomer.
Fluoroelastomers are recognized for their exceptional performance in harsh conditions, offering high-temperature resistance (up to 500°F or 260°C) and resistance to aggressive fluids, surpassing many other elastomers. They also demonstrate remarkable stability when exposed to various chemicals and fluids, including oil, diesel, ethanol blends, and bodily fluids.
The effectiveness of fluoroelastomers in resisting aggressive chemicals depends on the specific base polymer and the compounding ingredients used in the manufacturing process, such as for producing o-rings. While some formulations are generally compatible with hydrocarbons, they may be incompatible with substances like ketones (e.g., acetone and methyl ethyl ketone), ester solvents (e.g., ethyl acetate), amines, and organic acids like acetic acid.
One distinguishing characteristic of fluoroelastomers is their high density, exceeding 1800 kg/m³, which is significantly higher than most other types of rubber. This density is one of the factors that set them apart from many other elastomeric materials.
Pharmaceutical Uses: In pharmaceuticals, Viton™ is predominantly utilized for manufacturing seals, gaskets, and O-rings that are vital for maintaining the integrity of pharmaceutical processing equipment. Its robust chemical resistance ensures compatibility with a wide range of pharmaceutical substances, while its resilience to high temperatures and harsh sterilization processes is crucial for maintaining product purity.
Beyond pharmaceuticals, Viton™ (FKM) finds extensive use in automotive, aerospace, and industrial applications, where its unmatched combination of properties, including resistance to fuels and oils, make it a preferred choice for sealing solutions that require reliability and longevity.
Beyond pharmaceuticals, Viton™ (FKM) finds extensive use in automotive, aerospace, and industrial applications, where its unmatched combination of properties, including resistance to fuels and oils, make it a preferred choice for sealing solutions that require reliability and longevity.
|
|
The Center for Pharmaceutical Cleaning Innovation is a Non-Profit Research Organization providing research and educational opportunities in Cleaning Process Development and Validation
Content Copyright 2022. All rights reserved. [email protected] (908) 507-7743 |