Center for Pharmaceutical Cleaning Innovation
CPCI™ Coupon Cleaner
Surrogate surfaces, or “coupons” as they are commonly called, have been used for many years as a substitute for a piece of manufacturing equipment or a medical device surface (ASTM E3106) for performing swab/rinse recovery studies. Today these coupons are also used for Cleanability Testing, Disinfectant Testing and for creating Visual Coupon Standards for the qualification of Visual Inspection.
ASTM G121 “Standard Practice Guide for Preparation of Contaminated Test Coupons for the Evaluation of Cleaning Agents” advises that test coupons should be cleaned after use with a defined procedure that restores the test coupons’ surfaces to their original state and that the test coupons must be dried and stored until needed to avoid possible contamination. Test coupons should be examined prior to use to determine if they are acceptable for use against a defined standard for the test coupons, for example, change in surface roughness (Ra), maximum number of scratches, surface imperfections that may interfere with evaluation, staining, lack of visual cleanliness, etc.).
It has been a common practice for coupons to be stored in a lab drawer mixed in with other coupons where the coupons may be damaged or contaminated or mixed up with other coupons. When stored together, coupons of one Material of Construction that are unmarked or unlabeled may be easily confused with coupons of similar Materials of Construction and incorrectly selected for a study.
CPCI™ has developed the following procedures for cleaning, handling and storing of these coupons.
ASTM G121 “Standard Practice Guide for Preparation of Contaminated Test Coupons for the Evaluation of Cleaning Agents” advises that test coupons should be cleaned after use with a defined procedure that restores the test coupons’ surfaces to their original state and that the test coupons must be dried and stored until needed to avoid possible contamination. Test coupons should be examined prior to use to determine if they are acceptable for use against a defined standard for the test coupons, for example, change in surface roughness (Ra), maximum number of scratches, surface imperfections that may interfere with evaluation, staining, lack of visual cleanliness, etc.).
It has been a common practice for coupons to be stored in a lab drawer mixed in with other coupons where the coupons may be damaged or contaminated or mixed up with other coupons. When stored together, coupons of one Material of Construction that are unmarked or unlabeled may be easily confused with coupons of similar Materials of Construction and incorrectly selected for a study.
CPCI™ has developed the following procedures for cleaning, handling and storing of these coupons.
Cleaning of Coupons
Even if the previous study results show nearly 100% recovery, unless the coupons are carefully cleaned, there will still be some material remaining on the coupons which can significantly alter the recovery results of the next study that uses the same coupons. Therefore, all coupons must be scrupulously cleaned before each study.
CPCI™ has developed a Coupon Cleaner that consists of both a mild abrasive and a powerful cleaning agent that do not contain any organic carbon compounds. The components are non-hazardous - they are listed as SCOGS Type 1 (There is no evidence in the available information on the substance that demonstrates, or suggests reasonable grounds to suspect, a hazard to the public when they are used at levels that are now current or might reasonably be expected in the future) in the FDA's GRAS (Generally Recognized As Safe) Database. Using the CPCI™ Coupon Cleaner will physically and chemically remove all residues from the coupons and leave no traces of organic carbon.
The following simple and easy procedure is recommended:
CPCI™ has developed a Coupon Cleaner that consists of both a mild abrasive and a powerful cleaning agent that do not contain any organic carbon compounds. The components are non-hazardous - they are listed as SCOGS Type 1 (There is no evidence in the available information on the substance that demonstrates, or suggests reasonable grounds to suspect, a hazard to the public when they are used at levels that are now current or might reasonably be expected in the future) in the FDA's GRAS (Generally Recognized As Safe) Database. Using the CPCI™ Coupon Cleaner will physically and chemically remove all residues from the coupons and leave no traces of organic carbon.
The following simple and easy procedure is recommended:
1 - Pipette approximately 0.5 mL of Low TOC Water onto the surface of the coupon
2 - Sprinkle about 0.5 gram of CPCI™ Coupon Cleaner onto the coupon
3 - Firmly rub the test area of the coupon in back-and-forth and up-and-down patterns with a non-shedding wipe.
4 - Rinse the coupon with Low TOC Water (do not use tap water as it is typically very high in TOC and will leave organic carbon residues on the coupon.
This procedure will leave the coupon's test area free of any organic carbon residues resulting in low coupon blank results and reliable TOC Swab/Rinse Recovery study results.
This procedure will leave the coupon's test area free of any organic carbon residues resulting in low coupon blank results and reliable TOC Swab/Rinse Recovery study results.
Handling of Coupons
- Gloves should be worn during sample handling to prevent inadvertent contamination through fingerprints that can lead to biased recovery results.
- Gloves should be rinsed with Low TOC Water prior to handling coupons.
- Inspect coupons for defects (e.g., scratches) that may interfere with the recovery study. If a coupon is defective it should be replaced.
- There should be no talking during recovery studies or during validation sampling to prevent small drops of saliva from contaminating the coupons or the samples.
- Wearing a mask during recovery studies or during validation sampling is recommended.
Storing of Coupons
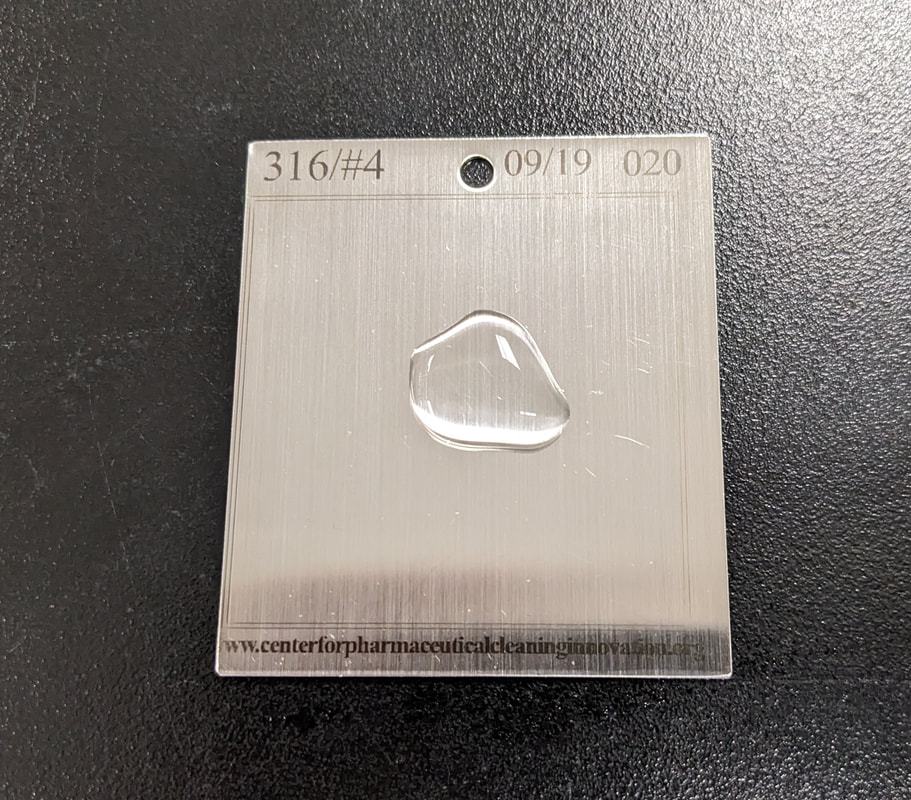
Proper care of coupons will provide many years of successful and reliable use from the coupons. Coupons must be stored in a protective container to prevent damage to, or contamination of, the coupons. CPCI™ provides a convenient storage box with all coupons purchased from CPCI™. The coupons are engraved with the Material of Construction for easy confirmation of the correct coupons prior to performing a study. The date of manufacture is also engraved on the coupon that can be used in an inspection program for defects in the coupons through their ongoing use.
Example of CPCI™ Coupons with Storage Boxes
|
|
The Center for Pharmaceutical Cleaning Innovation is a Non-Profit Research Organization providing research and educational opportunities in Cleaning Process Development and Validation
Content Copyright 2022. All rights reserved. [email protected] (908) 507-7743 |