Center for Pharmaceutical Cleaning Innovation
Cleaning Process Development
|
CPCI™ is a pioneer in the area of Cleaning Process Development having devoted many years of research into developing new methods and new technologies (patents pending) that have resulted in rapid and cost-effective new approaches that are truly Science, Risk and Statistics-based. These techniques are particularly important in Risk Reduction efforts when the Risk Analysis or Risk Evaluation shows that the Risk to patient safety is too high.
|
Bench Scale Cleaning Process Development |
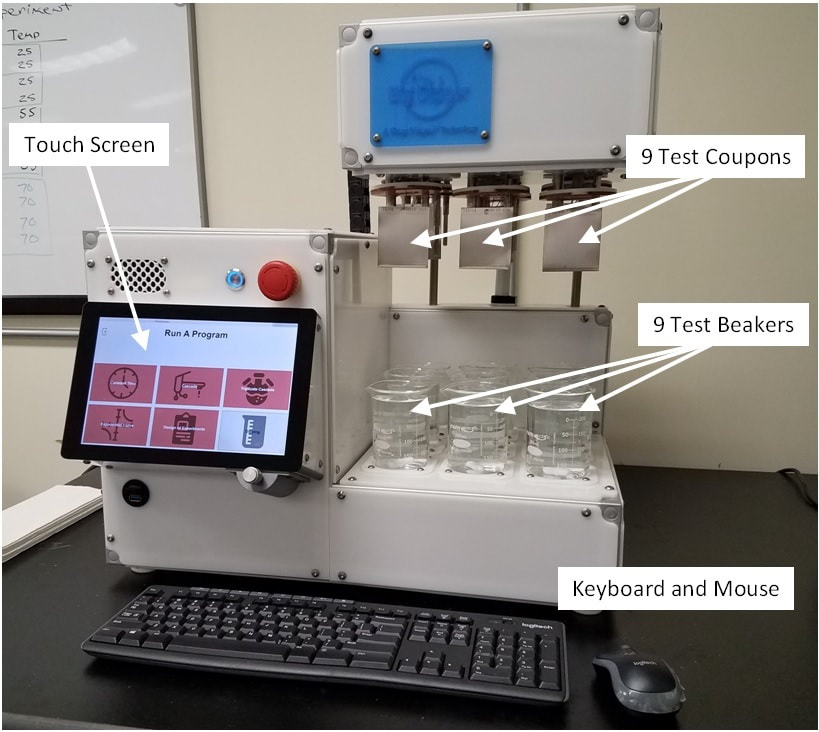
CPCI™ has developed laboratory instruments for evaluating compounds/products, following the ASTM G121 Standard Practice and the ASTM G122 Standard Method, to compare their ability to be cleaned to other compounds/products so as to create a ranking system for determining "hardest-to-clean" compounds/products using a standard procedure or a customized procedure for your cleaning process. These same procedures can be used to screen Cleaning Agents to determine the best Cleaning Agent for your compound/product or to compare Cleaning Agents to show equivalency for Cleaning Validation "bridging" studies. Equivalency studies can be used to justify reducing or even eliminating Cleaning Validations (3X runs) for New Products.
See our articles on: Determining "Hardest to Clean" Products Cleaning Agent Selection "Time to Clean" Studies and the "Cleaning Assurance Level" Using Design of Experiments in Cleaning Validation |
Full Scale Cleaning Process Development
|
After the Bench Scale studies have selected the appropriate Cleaning Agent and narrowed down the parameters, a strategy for executing Full Scale Studies can be implemented quickly and easily with a much greater chance of 1st time success along with a much lower chance for any cleaning failures.
|
|
|
The Center for Pharmaceutical Cleaning Innovation is a Non-Profit Research Organization providing research and educational opportunities in Cleaning Process Development and Validation
Content Copyright 2022. All rights reserved. [email protected] (908) 507-7743 |