Center for Pharmaceutical Cleaning Innovation
About CPCI™

CPCI™ is a not-for-profit research organization created by Andrew Walsh.
CPCI™ Vision is to become the top provider of research, education and consulting in Cleaning Process Development and Cleaning Validation for Pharmaceutical, Biotech, Cosmetics and Medical Device companies.
CPCI™ Mission is to enable the implementation of the American Society for Testing and Materials (ASTM) E3106-17 Standard Guide to "Science-Based and Risk-Based Cleaning Process Development and Validation" through our work on publishing additional ASTM Cleaning Standards, through our research and the development of new patented technologies, and through our publications, presentations, and our educational offerings.
CPCI™, through Andrew, has been involved with several industry initiatives concerning Cleaning Validation and was an author of ISPE’s Risk-based Manufacture of Pharmaceutical Products Guideline (Risk-MaPP) and has led the ASTM teams that wrote the:
and are currently working on several more proposed Standards (Work Items):
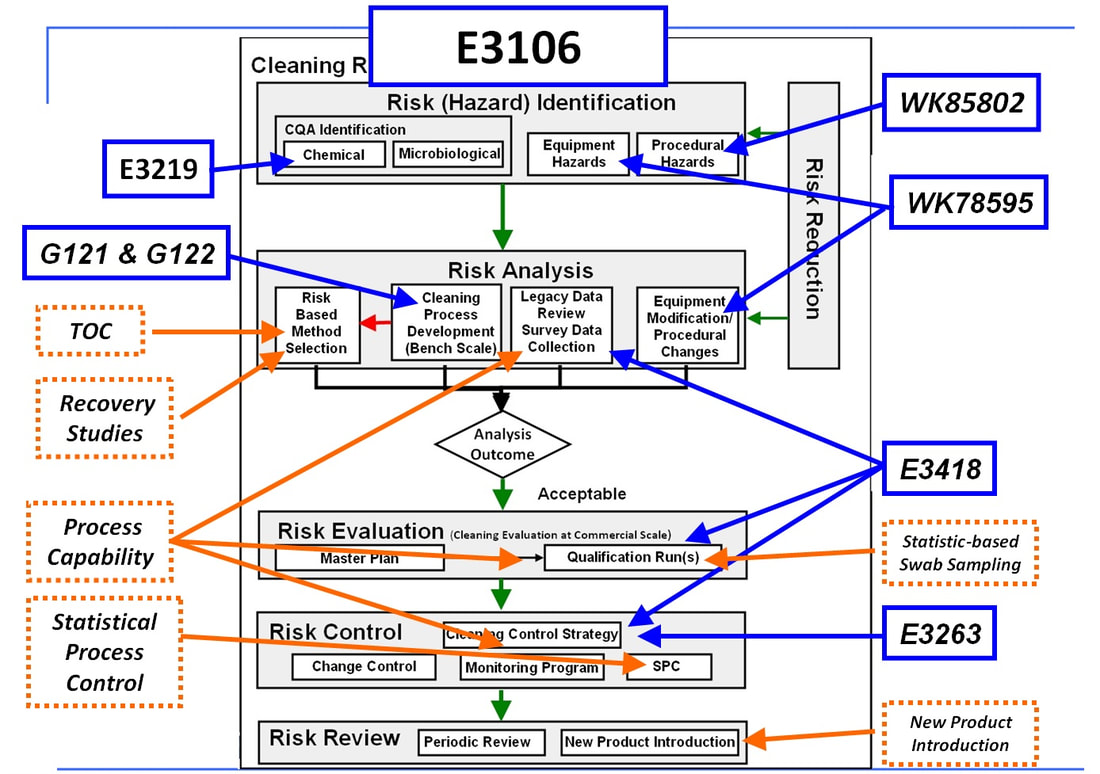
The figure below of the Cleaning Risk Management Process of E3106 shows these Standards and Work Items (shown in blue boxes) and where they apply.
CPCI™ Vision is to become the top provider of research, education and consulting in Cleaning Process Development and Cleaning Validation for Pharmaceutical, Biotech, Cosmetics and Medical Device companies.
CPCI™ Mission is to enable the implementation of the American Society for Testing and Materials (ASTM) E3106-17 Standard Guide to "Science-Based and Risk-Based Cleaning Process Development and Validation" through our work on publishing additional ASTM Cleaning Standards, through our research and the development of new patented technologies, and through our publications, presentations, and our educational offerings.
CPCI™, through Andrew, has been involved with several industry initiatives concerning Cleaning Validation and was an author of ISPE’s Risk-based Manufacture of Pharmaceutical Products Guideline (Risk-MaPP) and has led the ASTM teams that wrote the:
- ASTM E3106-17 "Standard Guide for Science-based and Risk-based Cleaning Process Development and Validation"
- ASTM E3109 "Standard Guide for Derivation of Health-Based Exposure Limits (HBELs)"
- ASTM’s G121 “Standard Practice for Preparation of Contaminated Test Coupons for the Evaluation of Cleaning Agents" update to include pharmaceuticals and Medical Devices
- ASTM G122 “Standard Test Method for Evaluating the Effectiveness of Cleaning Agents and Processes" update to include pharmaceuticals and Medical Devices
- ASTM E3263 “Standard Practice for Qualification for Visual Inspection of Pharmaceutical Manufacturing Equipment and Medical Devices for Residues"
- ASTM E3418 "Standard Practice for Calculating Scientifically Justifiable Limits of Residues for Cleaning of Pharmaceutical and Medical Device Manufacturing Equipment and for Medical Devices"
and are currently working on several more proposed Standards (Work Items):
- ASTM WK80508 "Standard Guide for Using FMECA and HACCP for Performing Risk Identification, Risk Analysis & Risk Control on Cleaning Processes for Pharmaceuticals and Medical Devices"
- ASTM WK78595 "Standard Guide for the Design of Clean in Place-Friendly Equipment for Pharmaceutical and Biopharmaceutical Applications (CbD Clean by Design)"
The figure below of the Cleaning Risk Management Process of E3106 shows these Standards and Work Items (shown in blue boxes) and where they apply.
Future Work Items will include Using Total Organic Carbon (TOC), Performing Recovery Studies, Calculation of Process Capability, Statistical Process Control, Statistics-based Swab Sampling and New Product Introduction (shown in orange boxes above).
About Andrew Walsh
Prior to joining the Pharmaceutical industry Andrew worked for the Colgate-Palmolive and Clorox companies as an Analytical Chemist and Microbiologist for 10 years where he gained insight into detergent chemistry, analytical methods (wet and instrumental) including method development manufacturing processes and statistical quality control.
Andrew then gained over 25 years of "hands-on" Validation experience working for Pharmaceutical and Biologics companies such as Johnson & Johnson companies RWJ Pharmaceutical Research Institute, Ortho-McNeil and Orth-Biotech (10 years), Schering-Plough (1 year) and Hoffmann-La Roche (5 years). Andrew's Validation and Qualification experience ranges from Equipment and Utility Qualification to Cleaning Validation to Process Validation to Spreadsheet and Computer Systems Validation.
During this time, Andrew gained experience in all aspects of Cleaning Validation; including writing policies and masterplans, developing cleaning procedures, writing and executing protocol and developing reports, calculating acceptance limits, developing and validating TOC and HPLC methods and developing and qualifying Visual Inspection. Andrew has presented on all of these topics for over 20 years at conferences with organizations such as Barnett, IIR, IPA, IVT, ISPE, Patheon, PharmaEd, KENX and also with the FDA.
After leaving Hoffmann-La Roche in 2007, Andy founded a consulting company, Clean6Sigma, LLC and has been providing training and consulting using Lean and Six Sigma techniques to large and small pharmaceutical companies including Actavis, Bristol-Meyers Squibb, C.R. Bard, Novartis and Johnson and Johnson. In 2012, Andy decided to merge Clean6Sigma, LLC with Rocky Mountain Analytical to create PharmaClean Group, LLC. The PharmaClean Group was dissolved on December 31st 2015. The "Center for Pharmaceutical Cleaning Innovation" (CPCI) was officially incorporated as a not-for-profit organization and started on January 1st 2016 and operates a research and educational laboratory in Hillsborough, NJ.
Andrew was also an Industry Professor from 2008 - 2015 in the Pharmaceutical Manufacturing and Engineering Graduate Program at Stevens Institute of Technology where he created and taught courses in Pharmaceutical Validation and Lean Six Sigma. While at Stevens, Andrew also founded and directed the Stevens Pharmaceutical Research Center (SPRC) from 2009 - 2015 which focused on Cleaning and Cleaning Validation topics
Prior to joining the Pharmaceutical industry Andrew worked for the Colgate-Palmolive and Clorox companies as an Analytical Chemist and Microbiologist for 10 years where he gained insight into detergent chemistry, analytical methods (wet and instrumental) including method development manufacturing processes and statistical quality control.
Andrew then gained over 25 years of "hands-on" Validation experience working for Pharmaceutical and Biologics companies such as Johnson & Johnson companies RWJ Pharmaceutical Research Institute, Ortho-McNeil and Orth-Biotech (10 years), Schering-Plough (1 year) and Hoffmann-La Roche (5 years). Andrew's Validation and Qualification experience ranges from Equipment and Utility Qualification to Cleaning Validation to Process Validation to Spreadsheet and Computer Systems Validation.
During this time, Andrew gained experience in all aspects of Cleaning Validation; including writing policies and masterplans, developing cleaning procedures, writing and executing protocol and developing reports, calculating acceptance limits, developing and validating TOC and HPLC methods and developing and qualifying Visual Inspection. Andrew has presented on all of these topics for over 20 years at conferences with organizations such as Barnett, IIR, IPA, IVT, ISPE, Patheon, PharmaEd, KENX and also with the FDA.
After leaving Hoffmann-La Roche in 2007, Andy founded a consulting company, Clean6Sigma, LLC and has been providing training and consulting using Lean and Six Sigma techniques to large and small pharmaceutical companies including Actavis, Bristol-Meyers Squibb, C.R. Bard, Novartis and Johnson and Johnson. In 2012, Andy decided to merge Clean6Sigma, LLC with Rocky Mountain Analytical to create PharmaClean Group, LLC. The PharmaClean Group was dissolved on December 31st 2015. The "Center for Pharmaceutical Cleaning Innovation" (CPCI) was officially incorporated as a not-for-profit organization and started on January 1st 2016 and operates a research and educational laboratory in Hillsborough, NJ.
Andrew was also an Industry Professor from 2008 - 2015 in the Pharmaceutical Manufacturing and Engineering Graduate Program at Stevens Institute of Technology where he created and taught courses in Pharmaceutical Validation and Lean Six Sigma. While at Stevens, Andrew also founded and directed the Stevens Pharmaceutical Research Center (SPRC) from 2009 - 2015 which focused on Cleaning and Cleaning Validation topics
Andrew is an Adjunct Associate Professor at Temple University (2019 - present) and teaches the "Cleaning Validation" course as part of their Regulatory Affairs and Quality Assurance Program.
Andrew has a B.S. in Biology and an M.S. in Biology (specializing in Microbiology) and is also a certified Lean Six Sigma Black Belt (license # GR7764000076AW) and an Accredited Lean Six Sigma Trainer.
Andrew has a B.S. in Biology and an M.S. in Biology (specializing in Microbiology) and is also a certified Lean Six Sigma Black Belt (license # GR7764000076AW) and an Accredited Lean Six Sigma Trainer.
|
|
The Center for Pharmaceutical Cleaning Innovation is a Non-Profit Research Organization providing research and educational opportunities in Cleaning Process Development and Validation
Content Copyright 2022. All rights reserved. [email protected] (908) 507-7743 |