Center for Pharmaceutical Cleaning Innovation
CPCI can also provide "fee for service" technical and laboratory support to you on a wide variety of Cleaning Development and Cleaning Validation activities.
|
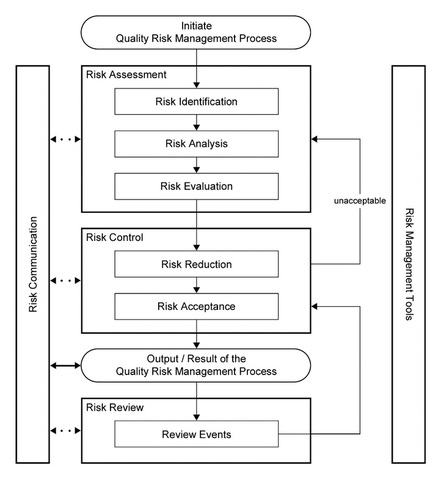
From ICH Q9 Quality Risk Management at http://www.ich.org/products/guidelines/quality/quality-single/article/quality-risk-management.html
|
CPCI's Risk-based Approach to Cleaning Validation
CPCI is a strong proponent of the International Congress on Harmonization (ICH) Q9 Quality Risk Management Process approach and uses ICH Q9 for determining the levels of Risk in all aspects of our Cleaning projects. All Cleaning Processes should have a comprehensive, science-based Risk Assessment performed where a evaluation of risk that includes not only evaluation of Chemical Hazards (APIs, Cleaning Agents, degradants, etc.) and potential Microbiological Hazards (microorganisms, endotoxins, etc.) but also potential Equipment Design Hazards and Procedural Hazards (SOPs, etc.). With this approach, all possible risks to patient safety and product quality can be identified and remediated if necessary before there is an issue.
Also with this approach, all the work in the cleaning program is driven by the level of risk; so for high risk situations, the level of effort and documentation may be significant and result in safer medicines (to the patients' benefit). For low risk situations, the level of effort and documentation can be much lower and result in a significant cost avoidance (to the manufacturers' benefit).
Also with this approach, all the work in the cleaning program is driven by the level of risk; so for high risk situations, the level of effort and documentation may be significant and result in safer medicines (to the patients' benefit). For low risk situations, the level of effort and documentation can be much lower and result in a significant cost avoidance (to the manufacturers' benefit).
Hazard Identification
CPCI has pioneered an approach that utilizes certified Toxicologist determined PDEs/ADEs for compounds that present a potential hazard coupled with our database of existing Safety Assessments on a wide array of common and known safe compounds to focus Cleaning Validation efforts only on relevant and truly potential hazards. Equipment Design Hazards and Procedural Hazards (SOPs) are also identified.
|
Risk Analysis
The Risk of the identified Hazards is the assessed by analyzing the potential of exposure through determination of the Maximum Safe Surface Residue (MSSR) and comparison to existing or historical data and Cleaning Process knowledge and understanding. If the Risk is acceptable then a Cleaning Validation (Risk Evaluation) is scheduled. If the Risk is high, then Risk Reduction steps are called for prior to Risk Evaluation.
|
Risk Evaluation
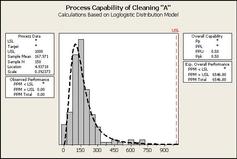
Risk Evaluation compares the Risk Analysis results with actual Cleaning Validation data to determine if the Risk is indeed acceptable. The best approach to do this is to perform a Process Capability analysis on the Cleaning Validation data (swab results, etc.) using the MSSR as the acceptance criteria. This simple statistical technique can quantify the level of Risk and also allow the prediction of the possibility of Cleaning Process failures in the future.
|
Risk Control
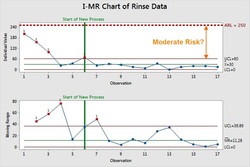
After the Risk Evaluation has been successful it is now important to determine an "Control Strategy" and the degree of any ongoing or periodic monitoring that will be used. The Process Capability results can be used as a guide - low Cpk values require monitoring, high Cpk values require less or none. Also, this is the appropriate stage to begin determination of Statistical Process Control (SPC) limits for the Cleaning Process.
|
|
|
The Center for Pharmaceutical Cleaning Innovation is a Non-Profit Research Organization providing research and educational opportunities in Cleaning Process Development and Validation
Content Copyright 2022. All rights reserved. [email protected] (908) 507-7743 |